Issue published October 1, 2025 Previous issue

- Volume 135, Issue 19
Go to section:
On the cover: Circadian rhythmicity of chronic pain
Taccardi, Zacharias, Gowdy, et al. report that circadian rhythms in pain intensity and immune cell profiles are indicators of reduced opioid use and improved biopsychosocial characteristics, while arrhythmic pain profiles reflect poor outcomes. The painting on the cover represents the potential for improving pain relief by restoring circadian rhythmicity. Image credit: Quána Madison.
In-Press Preview - More
Abstract
The intratumor microenvironment shapes the metastatic potential of cancer cells and their susceptibility to any immune response. Yet the nature of the signals within the microenvironment that control anti-cancer immunity and how they are regulated is poorly understood. Here, using melanoma as a model, we investigate the involvement in metastatic dissemination and the immune-modulatory microenvironment of Protein S-Acyl Transferases, as an underexplored class of potential therapeutic targets. We find that ZDHHC13, suppresses metastatic dissemination by palmitoylation of CTNND1, leading to stabilization of E-cadherin. Importantly, ZDHHC13 also reshapes the tumor immune microenvironment by suppressing lysophosphatidylcholine (LPC) synthesis in melanoma cells, leading to inhibition of M2-like tumor-associated macrophages that we show degrades E-cadherin via MMP12 expression. Consequently, ZDHHC13 activity suppresses tumor growth and metastasis in immunocompetent mice. Our study highlights the therapeutic potential of targeting the ZDHHC13-E-cadherin axis and its downstream metabolic and immune-modulatory mechanisms, offering additional strategies to inhibit melanoma progression and metastasis.
Authors
Hongjin Li, Jianke Lyu, Yu Sun, Chengqian Yin, Yuewen Li, Weiqiang Chen, Suan-Sin Foo, Xianfang Wu, Colin Goding, Shuyang Chen
Abstract
Deficits in the mitochondrial energy-generating machinery cause mitochondrial disease (MD), a group of untreatable and usually fatal disorders. Among many severe symptoms, refractory epileptic events are a common neurological presentation of MD. However, the neuronal substrates and circuits for MD-induced epilepsy remain unclear. Here, using mouse models of Leigh Syndrome, a severe form of MD associated to epilepsy, that lack mitochondrial complex I subunit NDUFS4 in a constitutive or conditional manner, we demonstrate that mitochondrial dysfunction leads to a reduction in the number of GABAergic neurons in the rostral external globus pallidus (GPe), and identify a specific affectation of pallidal Lhx6-expressing inhibitory neurons, contributing to altered GPe excitability. Our findings further reveal that viral vector-mediated Ndufs4 re-expression in the GPe effectively prevents seizures and improves the survival in the models. Additionally, we highlight the subthalamic nucleus (STN) as a critical structure in the neural circuit involved in mitochondrial epilepsy, as its inhibition effectively reduces epileptic events. Thus, we have identified a role for pallido-subthalamic projections in the development of epilepsy in the context of mitochondrial dysfunction. Our results suggest STN inhibition as a potential therapeutic intervention for refractory epilepsy in patients with MD providing promising leads in the quest to identify effective treatments.
Authors
Laura Sánchez-Benito, Melania González-Torres, Irene Fernández-González, Laura Cutando, María Royo, Joan Compte, Miquel Vila, Sandra Jurado, Elisenda Sanz, Albert Quintana
Abstract
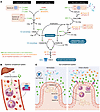
Single-cell studies have revealed that intestinal macrophages maintain gut homeostasis through the balanced actions of reactive (inflammatory) and tolerant (non-inflammatory) subpopulations. How such balance is impaired in inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), remains unresolved. Here, we define colon-specific macrophage states and reveal the critical role of non-inflammatory colon-associated macrophages (niColAMs) in IBD recovery. Through trans-scale analyses—integrating computational transcriptomics, proteomics, and in vivo interventional studies—we identified GIV (CCDC88A) as a key regulator of niColAMs. GIV emerged as the top-ranked gene in niColAMs that physically and functionally interacts with NOD2, an innate immune sensor implicated in CD and UC. Myeloid-specific GIV depletion exacerbates infectious colitis, prolongs disease, and abolishes the protective effects of the NOD2 ligand, muramyl dipeptide, in colitis and sepsis models. Mechanistically, GIV’s C-terminus binds the terminal leucine-rich repeat (LRR#10) of NOD2 and is required for NOD2 to dampen inflammation and clear microbes. The CD-associated 1007fs NOD2-variant, which lacks LRR#10, cannot bind GIV—providing critical insights into how this clinically relevant variant impairs microbial sensing and clearance. These findings illuminate a critical GIV-NOD2 axis essential for gut homeostasis and highlight its disruption as a driver of dysbiosis and inflammation in IBD.
Authors
Gajanan D. Katkar, Mahitha Shree Anandachar, Stella-Rita C. Ibeawuchi, Ella G. McLaren, Megan L. Estanol, Kennith Carpio-Perkins, Shu-Ting Hsu, Celia R. Espinoza, Jane E. Coates, Yashaswat S. Malhotra, Madhubanti Mullick, Vanessa Castillo, Daniella Vo, Saptarshi Sinha, Pradipta Ghosh
Abstract
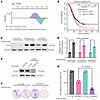
TMPRSS2:ERG gene fusion (T:E fusion) in prostate adenocarcinoma (PCa) puts ERG under androgen receptor (AR) regulated TMPRSS2 expression. T:E fusion is associated with PTEN loss, and is highly associated with decreased INPP4B expression, which together may compensate for ERG-mediated suppression of AKT signaling. We confirmed in PCa cells and a mouse PCa model that ERG suppresses IRS2 and AKT activation. In contrast, ERG downregulation did not increase INPP4B, suggesting its decrease is indirect and reflects selective pressure to suppress INPP4B function. Notably, INPP4B expression is decreased in PTEN-intact and PTEN-deficient T:E fusion tumors, suggesting selection for a nonredundant function. As ERG in T:E fusion tumors is AR regulated, we further assessed whether AR inhibition increases AKT activity in T:E fusion tumors. A T:E fusion positive PDX had increased AKT activity in vivo and response to AKT inhibition in vitro after androgen deprivation. Moreover, two clinical trials of neoadjuvant AR inhibition prior to radical prostatectomy showed greater increases in AKT activation in the T:E fusion positive versus negative tumors. These findings indicate that AKT activation may mitigate the efficacy of AR targeted therapy in T:E fusion PCa, and that these patients may most benefit from combination therapy targeting AR and AKT.
Authors
Fen Ma, Sen Chen, Luigi Cecchi, Betul Ersoy-Fazlioglu, Joshua W. Russo, Seiji Arai, Seifeldin Awad, Carla Calagua, Fang Xie, Larysa Poluben, Olga Voznesensky, Anson T. Ku, Fatima Karzai, Changmeng Cai, David J. Einstein, Huihui Ye, Xin Yuan, Alex Toker, Mary-Ellen Taplin, Adam G. Sowalsky, Steven P. Balk
Abstract
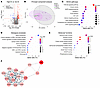
Immune cells are constantly exposed to microbiota-derived compounds that can engage innate recognition receptors. How this constitutive stimulation is down-modulated to avoid systemic inflammation and auto-immunity is poorly understood. Here we show that Aryl hydrocarbon Receptor (AhR) deficiency in monocytes unleashes spontaneous cytokine responses in vivo, driven by STING-mediated tonic sensing of microbiota. This effect was specific to monocytes, as mice deficient for AhR specifically in macrophages did not show any dysregulation of tonic cytokine responses. AhR inhibition also increased tonic cytokine production in human monocytes. Finally, in patients with systemic juvenile idiopathic arthritis, low AhR activity in monocytes correlated with elevated cytokine responses. Our findings evidence an essential role for AhR in monocytes in restraining tonic microbiota sensing and in maintaining immune homeostasis.
Authors
Adeline Cros, Alessandra Rigamonti, Alba de Juan, Alice Coillard, Mathilde Rieux-Laucat, Darawan Tabtim-On, Emeline Papillon, Christel Goudot, Alma-Martina Cepika, Romain Banchereau, Virginia Pascual, Marianne Burbage, Burkhard Becher, Elodie Segura
View more articles by topic:
Sign up for email alerts
Review Series - More
Series edited by Ben Z. Stanger
Pancreatic Cancer
Series edited by Ben Z. Stanger
Pancreatic ductal adenocarcinoma (PDAC) has among the poorest prognosis and highest refractory rates of all tumor types. The reviews in this series, by Dr. Ben Z. Stanger, bring together experts across multiple disciplines to explore what makes PDAC and other pancreatic cancers so distinctively challenging and provide an update on recent multipronged approaches aimed at improving early diagnosis and treatment.
×