Review
Abstract
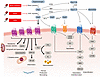
The renin-angiotensin-aldosterone system (RAAS) is a central regulator of cardiovascular, renal, and fluid homeostasis. Over the past century, our understanding of RAAS has evolved from a unidimensional circulatory hormone system to a complex network that includes local and intracellular signaling pathways. Aging profoundly impacts this system, influencing both systemic and tissue-specific RAAS activity. While levels of systemic RAAS components, such as plasma renin and aldosterone, decline with age, local RAAS components, particularly the proinflammatory angiotensin (Ang)II/AngII type 1 receptor (AT1R) axis, are upregulated in aging tissues, contributing to vasoconstriction, oxidative stress, inflammation, and fibrosis. Conversely, the protective arms of RAAS, the AngII/AT2R and Ang-(1–7)/Mas receptor pathways, are downregulated. Recent advances in geroscience have further illuminated how RAAS intersects with fundamental aging mechanisms, providing a mechanistic framework for understanding RAAS not only as a driver of age-related disease but also as a modifiable contributor to the aging process itself. In this Review, we summarize the evolution of RAAS biology, examine the molecular and functional consequences of aging on RAAS activity, and discuss the translational relevance of these findings. Finally, we explore emerging therapeutic strategies targeting RAAS components as potential interventions to promote healthy aging and reduce age-related disease burden, emphasizing a translational arc moving from bedside to bench and back, with the ultimate goal of improving patient outcomes.
Authors
Caglar Cosarderelioglu, Peter M. Abadir
Abstract
Therapies based on glucagon-like peptide-1 (GLP-1) reduce rates of cardiovascular and chronic kidney disease in people with type 2 diabetes and/or obesity, with ongoing clinical trials investigating their effects in people with metabolic liver disease, arthritis, and both substance use and neurodegenerative disorders. Acute and chronic activation of GLP-1 receptor signaling also reduces systemic and tissue inflammation in mice and humans, through weight loss–dependent and –independent mechanisms, actions that may contribute to the expanding spectrum of clinical benefits ascribed to GLP-1 medicines. In this Review, we highlight current understanding of the direct and indirect antiinflammatory effects and mechanisms of GLP-1 medicines in both preclinical and clinical studies, covering emerging concepts, clinical relevance, and areas of uncertainty that require further investigation.
Authors
Chi Kin Wong, Daniel J. Drucker
Abstract
Glucagon-like peptide-1 (GLP-1) was initially considered to be a hormone with a predominant role in regulating glucose metabolism by inducing insulin secretion, reducing glucagon secretion, and ameliorating insulin resistance, with the last effect being largely dependent on the induction of weight loss. In more recent years, the role of this peptide beyond metabolism has progressively been explored, including its impact on kidney physiology and kidney clinical outcomes in people with obesity with or without diabetes. Indeed, despite only modest expression of the GLP-1 receptor in the kidney, the renoprotective actions of GLP-1 and its receptor agonists have become an area of intensive investigation. This Review appraises the current status of GLP-1 peptide and its receptor agonists and focuses on the preclinical as well as recent seminal clinical findings defining the kidney benefits conferred by GLP-1 receptor agonist treatment in people living with type 2 diabetes and obesity.
Authors
Mark E. Cooper, Daniël H. van Raalte
Abstract
Cancer diagnoses are prevalent in people with obesity and type 2 diabetes, and abundant clinical evidence supports the protective effects of weight loss for cancer prevention. Glucagon-like peptide-1 (GLP-1) receptor agonists have revolutionized obesity and type 2 diabetes medicine and alleviate many comorbidities of these metabolic diseases. In this Review, we summarize the current clinical evidence for GLP-1 receptor agonists and cancer risk, including thyroid, pancreatic, gastrointestinal, and hormone-dependent malignancies. With few exceptions, recent meta-analyses report that GLP-1 receptor therapies do not increase cancer incidence and may lower risk in some cases. Preclinical studies reinforce the anticancer effects of GLP-1 receptor therapies, even in non-obese models. However, there are still many opportunities for translational insight as the field grows. Immune-modulating effects of GLP-1 receptor agonists are reported in several preclinical cancer studies, which may reflect direct action on immune cells or result from improved metabolic function. We highlight ongoing clinical trials for GLP-1 receptor therapies in cancer patients, and offer considerations for preclinical studies, including perspectives on the timing and duration of GLP-1 receptor agonist treatment, concurrent use of standard anticancer therapies, and interpretation of models of cancer risk versus progression.
Authors
Estefania Valencia-Rincón, Rajani Rai, Vishal Chandra, Elizabeth A. Wellberg
Abstract
Peptidyl arginine deiminases (PADs) catalyze the conversion of arginine residues into peptidyl citrulline, a posttranslational modification known as protein citrullination (or arginine deimination). This process alters the charge of proteins from positive to neutral, thereby affecting their folding, stability, conformation, and function. PAD2 and PAD4 can translocate into the nucleus and citrullinate both cytoplasmic and nuclear proteins. In this Review, we focus on PAD2- and PAD4-mediated citrullination in immune cell subsets within the tumor microenvironment. We discuss how citrullination regulates immune cell function and tumor immunity and explore the potential of targeting citrullination as a strategy for cancer immunotherapy.
Authors
Michael R. Pitter, Weiping Zou
Abstract
The increasing recognition of a new category of encephalitides that occur in association with antibodies against neuronal surface proteins has prompted the use of terms like “autoimmune psychosis” and “autoimmune psychiatric disorders.” However, although psychosis and other psychiatric symptoms can occur in autoimmune encephalitides and systemic autoimmune diseases, evidence for a distinct psychiatric entity beyond these conditions is lacking. A particularly defining condition is anti-NMDA receptor encephalitis, which has been central to promoting concepts such as autoimmune psychosis and autoimmune psychiatric disorders. While anti-NMDA receptor encephalitis can resemble primary psychiatric conditions, certain clinical features often suggest the specific diagnosis. This Review traces the development of the autoimmune psychosis concept and examines the implications of framing it as a separate entity. We discuss leading theories of psychosis and the convergence of the NMDA receptor hypofunction/glutamate hypothesis with anti-NMDA receptor encephalitis mechanisms. The interest generated by such disorders has driven uncontrolled antibody testing in psychiatric populations, often neglecting pretest probability and favoring prevalence over diagnostic specificity. Finally, we highlight the main limitations of current approaches and propose directions for future research.
Authors
José Maria Cabrera-Maqueda, Jesús Planagumà, Mar Guasp, Josep Dalmau
Abstract
Pancreatic cancer (PC) is a devastating disease, due in part to its diagnosis frequently being made at an advanced stage. Ongoing efforts are aimed at identifying early-stage PC in high-risk individuals, as early detection leads to downstaging of PC and improvements in survival. However, there are a myriad of challenges that arise when trying to optimize PC early detection strategies, including selection of the appropriate high-risk individuals and selection of the test or combination of tests that should be performed. Here, we discuss the populations that are the strongest candidates for PC screening and review professional PC screening guidelines. We also summarize the current state of imaging techniques for early detection of PC and further review many studied biomarkers — ranging from nucleic acid targets, proteins, and the microbiome — to highlight the current state of the field and the challenges that remain in the years to come.
Authors
Michael J. Shen, Arsia Jamali, Bryson W. Katona
Abstract
Chronic organ disease is often complicated by fibrosis, the excessive accumulation of extracellular matrix, as a consequence of dysfunctional wound healing responses. Fibrosis progressively distorts tissue architecture and eventually leads to loss of organ function, accounting for up to 45% of deaths in developed countries. Moreover, fibrosis is a major risk factor for tumor development. The few approved therapies aimed at preventing or resolving fibrosis show limited efficacy and safety. One reason for the lack of efficient antifibrotic therapies is the fact that the cell circuits driving the disease biology are still only partially understood. The circadian clock is known to regulate the physiological functions of critical organs, including the liver, kidneys, and lungs. Several experimental and clinical studies have established that circadian disruption plays an important role in the development of chronic diseases across organs involving fibrosis. These include metabolic dysfunction–associated steatotic liver disease, chronic kidney disease, and chronic obstructive pulmonary disease. Here, we provide an overview of the circadian mechanisms that play critical roles in mediating physiological functions in the liver, kidneys, and lungs and whose deregulations could predispose toward development of chronic disease of these organs, leading to fibrosis. We also highlight the possible opportunities of chronotherapy for chronic diseases and discuss future perspectives.
Authors
Atish Mukherji, Pierre-Louis Tharaux, David W. Ray, Thomas F. Baumert
Abstract
The gut microbiota plays a crucial role in maintaining intestinal homeostasis and influencing various aspects of host physiology, including immune function. Recent advances have highlighted the emerging importance of the complement system, particularly the C3 protein, as a key player in microbiota-host interactions. Traditionally known for its role in innate immunity, the complement system is now recognized for its interactions with microbial communities within the gut, where it promotes immune tolerance and protects against enteric infections. This Review explores the gut complement system as a possibly novel frontier in microbiota-host communication and examines its role in shaping microbial diversity, modulating inflammatory responses, and contributing to intestinal health. We discuss the dynamic interplay between microbiota-derived signals and complement activation, with a focus on the C3 protein and its effect on both the gut microbiome and host immune responses. Furthermore, we highlight the therapeutic potential of targeting complement pathways to restore microbial balance and treat diseases such as inflammatory bowel disease and colorectal cancer. By elucidating the functions of the gut complement system, we offer insights into its potential as a target for microbiota-based interventions aimed at restoring intestinal homeostasis and preventing disease.
Authors
Xianbin Tian, Lan Zhang, Xinyang Qian, Yangqing Peng, Fengyixin Chen, Sarah Bengtson, Zhiqing Wang, Meng Wu
Abstract
The complement system has emerged as a critical regulator of intestinal homeostasis, inflammation, and cancer. In this Review, we explore the multifaceted roles of complement in the gastrointestinal tract, highlighting its canonical and noncanonical functions across intestinal epithelial and immune cells. Under homeostatic conditions, intestinal cells produce complement that maintains barrier integrity and modulates local immune responses, but complement dysregulation contributes to intestinal inflammation and promotes colon cancer. We discuss recent clinical and preclinical studies to provide a cohesive overview of how complement-mediated modulation of immune and nonimmune cell functions can protect or exacerbate inflammation and colon cancer development. The complement system plays a dual role in the intestine, with certain components supporting tissue protection and repair and others exacerbating inflammation. Intriguingly, distinct complement pathways modulate colon cancer progression and response to therapy, with novel findings suggesting that the C3a/C3aR axis constrains early tumor development but may limit antitumor immunity. The recent discovery of intracellular complement activation and tissue-specific complement remains vastly underexplored in the context of intestinal inflammation and colon cancer. Collectively, complement functions are context- and cell-type-dependent, acting both as a shield and a sword in intestinal diseases. Future studies dissecting the temporal and spatial dynamics of complement are essential for leveraging its potential as a biomarker and therapeutic in colon cancer.
Authors
Carsten Krieg, Silvia Guglietta
No posts were found with this tag.