Issue published August 1, 2025 Previous issue

- Volume 135, Issue 15
Go to section:
On the cover: Kidney-specific WNK1 enhances responsiveness to potassium
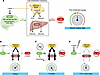
Boyd-Shiwarski et al. report that kidney-specific WNK1 is a scaffolding protein that organizes other proteins within biomolecular condensates called WNK bodies — structures essential for maintaining blood potassium and human health. The cover image shows immunofluorescence staining of WNK bodies (green) in the distal convoluted tubule (magenta) of a mouse kidney during hypokalemia. Image credit: Arohan Subramanya and Cary Boyd-Shiwarski.
In-Press Preview - More
Jab1 promotes immune evasion and progression in acute myeloid leukemia models under oxidative stress
Abstract
Acute myeloid leukemia (AML) is the most common hematological malignancy. Leukemia stem cells exhibit high levels of oxidative stress, with reactive oxygen species (ROS) being the primary products of this stress, inducing the expression of Jab1. Previous studies have demonstrated that Jab1, as a transcriptional coactivator of c-JUN, promotes the malignant progression of AML under oxidative stress. However, its role in immune evasion is still under investigation. Here, we observed that knocking out Jab1 reduced the expression of immune checkpoints in vivo, effectively overcame the immune evasion of AML. Interestingly, the deletion of Jab1 had no impact on the maturation of normal hematopoietic cells in mice. Mechanistically, Jab1 directly activated IGF2BP3 by driving the transcription factor c-JUN, consequently modulated the m6A modification of LILRB4 mRNA and promoted immune evasion in AML. Finally, CSN5i-3 effectively disrupted the signaling pathway mediated by Jab1, thereby restoring cellular immune surveillance and halting the progression of AML. Thus, our results highlight the functional role of Jab1 in supporting AML survival and support the development of targeted therapeutic strategies.
Authors
Nan Zhang, Qian Wang, Guopeng Chen, Li Liu, Zhiying Wang, Linlu Ma, Yuxing Liang, Jinxian Wu, Xinqi Li, Xiaoyan Liu, Fuling Zhou
Abstract
Aspergillus fumigatus is the most common cause of invasive aspergillosis (IA), a devastating infection in immunocompromised patients. Plasmacytoid dendritic cells (pDCs) regulate host defense against IA by enhancing neutrophil antifungal properties in the lung. Here, we define the pDC activation trajectory during A. fumigatus infection and the molecular events that underlie the protective pDC - neutrophil crosstalk. Fungus-induced pDC activation begins after bone marrow egress and results in pDC-dependent regulation of lung type I and type III IFN levels. These pDC-derived products act on type I and type III IFN receptor-expressing neutrophils and control neutrophil fungicidal activity and reactive oxygen species production via STAT1 signaling in a cell-intrinsic manner. Mechanistically, neutrophil STAT1 signaling regulates the transcription and expression of Cybb, which encodes one of five NADPH oxidase subunits. Thus, pDCs regulate neutrophil-dependent immunity against inhaled molds by controlling the local expression of a subunit required for NADPH oxidase assembly and activity in the lung.
Authors
Yahui Guo, Mariano A. Aufiero, Kathleen A.M. Mills, Simon A. Grassmann, Hyunu Kim, Mergim Gjonbalaj, Paul Zumbo, Audrey Billips, Katrina B. Mar, Yao Yu, Laura Echeverri Tirado, Lena Heung, Amariliz Rivera, Doron Betel, Joseph C. Sun, Tobias M. Hohl
Abstract
The leukemia fusion gene CBFB-MYH11 requires RUNX1 for leukemogenesis, but the underlying mechanism is unclear. By in vitro studies, we found that CBFβ-SMMHC, the chimeric protein encoded by CBFB-MYH11, could enhance the binding affinity between RUNX1 and its target DNA. Increased RUNX1-DNA binding was also observed in myeloid progenitor cells from mice expressing CBFβ-SMMHC. Moreover, only CBFβ-SMMHC variants able to enhance the DNA binding affinity by RUNX1 could induce leukemia in mouse models. Marked transcriptomic changes, affecting genes associated with inflammatory response and target genes of CBFA2T3, were observed in mice expressing leukemogenic CBFβ-SMMHC variants. Finally, we show that CBFβ-SMMHC could not induce leukemia in mice with a Runx1-R188Q mutation, which reduces RUNX1 DNA binding but not affecting its interaction with CBFβ-SMMHC or its sequestration to cytoplasm by CBFβ-SMMHC. Our data suggest that, in addition to binding RUNX1 to regulate gene expression, enhancing RUNX1 binding affinity to its target DNA is an important mechanism by which CBFβ-SMMHC contributes to leukemogenesis, highlighting RUNX1–DNA interaction as a potential therapeutic target in inv(16) AML.
Authors
Tao Zhen, Yaqiang Cao, Tongyi Dou, Yun Chen, Guadalupe Lopez, Ana Catarina Menezes, Xufeng Wu, John Hammer, Jun Cheng, Lisa Garrett, Stacie Anderson, Martha Kirby, Stephen Wincovitch, Bayu Sisay, Abdel G. Elkahloun, Di Wu, Lucio H. Castilla, Wei Yang, Jiansen Jiang, Keji Zhao, Paul P. Liu
Abstract
BRD4 is an epigenetic reader protein that regulates oncogenes such as myc in cancer. However, its additional role in shaping immune responses via regulation of inflammatory and myeloid cell responses is not yet fully understood. This work further characterized the multifaceted role of BRD4 in anti-tumor immunity. NanoString gene expression analysis of EMT6 tumors treated with a BRD4 inhibitor identified a reduction in myeloid gene expression signatures. Additionally, BRD4 inhibition significantly reduced myeloid derived suppressor cells (MDSC) in the spleens and tumors of mice in multiple tumor models and also decreased the release of tumor-derived MDSC growth and chemotactic factors. Pharmacologic inhibition of BRD4 in MDSC induced apoptosis and modulated expression of apoptosis regulatory proteins. A BRD4-myeloid specific knockout model suggested that the dominant mechanism of MDSC reduction after BRD4 inhibition was primarily through a direct effect on MDSC. BRD4 inhibition enhanced anti-PD-L1 therapy in the EMT6, 4T1, and LLC tumor models, and the efficacy of the combination treatment was dependent on CD8+ T cells and on BRD4 expression in the myeloid compartment. These results identify BRD4 as a regulator of MDSC survival and provide evidence to further investigate BRD4 inhibitors in combination with immune based therapies.
Authors
Himanshu Savardekar, Andrew Stiff, Alvin Liu, Robert Wesolowski, Emily Schwarz, Ian C. Garbarine, Megan C. Duggan, Sara Zelinskas, Jianying Li, Gabriella Lapurga, Alexander Abreo, Lohith Savardekar, Ryan Parker, Julia Sabella, Mallory J. DiVincenzo, Brooke Benner, Steven H. Sun, Dionisia Quiroga, Luke Scarberry, Gang Xin, Anup Dey, Keiko Ozato, Lianbo Yu, Merve Hasanov, Debasish Sundi, Richard C. Wu, Kari L. Kendra, William E. Carson III
Abstract
Mutant KRAS has been implicated in driving a quarter of all cancer types. Although inhibition of the KRASG12C mutant protein has shown clinical promise, there is still a need for therapies that overcome resistance and target non-KRASG12C mutations. KRAS activates downstream MYC, which is also a challenging-to-drug oncoprotein. We have developed an “inverted” RNAi molecule with the passenger strand of a MYC-targeting siRNA fused to the guide strand of a KRAS-targeting siRNA. The chimeric molecule simultaneously inhibits KRAS and MYC, showing marked improvements in efficacy beyond the individual siRNA components. This effect is mediated by 5’-dT overhangs following endosomal metabolism. The synergistic RNAi activity led to a >10-40-fold improvement in inhibiting cancer viability in vitro. When conjugated to an epidermal growth factor receptor (EGFR)-targeting ligand, the chimeric siRNA was delivered to and internalized by tumor cells. As compared with individual targeting siRNAs, the chimeric design resulted in considerably improved metabolic stability in tumors, enhanced silencing of both oncogenes, and reduced tumor progression in multiple cancer models. This inverted chimeric design establishes proof-of-concept for ligand-directed, dual-silencing of KRAS and MYC in cancer and constitutes an innovative molecular strategy for co-targeting any two genes of interest, which has broad implications.
Authors
Yogitha S Chareddy, Hayden P. Huggins, Snehasudha S Sahoo, Lyla Stanland, Christina Gutierrez-Ford, Kristina M. Whately, Lincy Edatt, Salma H Azam, Matthew C. Fleming, Jonah Im, Alessandro Porrello, Imani Simmons, Jillian L. Perry, Albert A. Bowers, Martin Egli, Chad V. Pecot
View more articles by topic:
Sign up for email alerts
Review Series - More
Series edited by Ben Z. Stanger
Pancreatic Cancer
Series edited by Ben Z. Stanger
Pancreatic ductal adenocarcinoma (PDAC) has among the poorest prognosis and highest refractory rates of all tumor types. The reviews in this series, by Dr. Ben Z. Stanger, bring together experts across multiple disciplines to explore what makes PDAC and other pancreatic cancers so distinctively challenging and provide an update on recent multipronged approaches aimed at improving early diagnosis and treatment.
×