Advertisement
Commentary
Open Access |  10.1172/JCI195258
10.1172/JCI195258
Integrin α4β7 as a predictor of HIV acquisition: one thread in a complex tapestry
Tosin E. Omole1,2 and Lyle R. McKinnon1,3,4
1Department of Medical Microbiology and Infectious Diseases, University of Manitoba, Winnipeg, Manitoba, Canada.
2Vaccine and Infectious Disease Organization (VIDO), Saskatoon, Saskatchewan, Canada.
3Centre for the AIDS Programme of Research in South Africa (CAPRISA), Durban, South Africa.
4Department of Medical Microbiology and Immunology, University of Nairobi, Nairobi, Kenya.
Address correspondence to: Lyle R. McKinnon, Department of Medical Microbiology and Infectious Diseases, 504-745 Bannatyne Avenue, Basic Medical Sciences Building, University of Manitoba, Winnipeg, Manitoba, R3E 0J9, Canada. Phone: 204.975.7708; Email: Lyle.Mckinnon@umanitoba.ca.
Find articles by Omole, T. in: PubMed | Google Scholar
1Department of Medical Microbiology and Infectious Diseases, University of Manitoba, Winnipeg, Manitoba, Canada.
2Vaccine and Infectious Disease Organization (VIDO), Saskatoon, Saskatchewan, Canada.
3Centre for the AIDS Programme of Research in South Africa (CAPRISA), Durban, South Africa.
4Department of Medical Microbiology and Immunology, University of Nairobi, Nairobi, Kenya.
Address correspondence to: Lyle R. McKinnon, Department of Medical Microbiology and Infectious Diseases, 504-745 Bannatyne Avenue, Basic Medical Sciences Building, University of Manitoba, Winnipeg, Manitoba, R3E 0J9, Canada. Phone: 204.975.7708; Email: Lyle.Mckinnon@umanitoba.ca.
Find articles by McKinnon, L. in: PubMed | Google Scholar
Published August 1, 2025 - More info
J Clin Invest. 2025;135(15):e195258. https://doi.org/10.1172/JCI195258.
© 2025 Omole, et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Related article:
Abstract
We leveraged specimens from the RV217 prospective study that enrolled participants at high risk of HIV-1 acquisition to investigate how NK cells, conventional T cells, and unconventional T cells influence HIV-1 acquisition. We observed low levels of α4β7 expression on memory CD4+ T cells and invariant NK T (iNKT) cells, 2 cell types highly susceptible to HIV-1 infection, in highly exposed seronegative (HESN) compared with highly exposed seroconverter (HESC) participants. NK cells from HESN individuals had higher levels of α4β7 than did those from HESC individuals, presented a quiescent phenotype, and had a higher capacity to respond to opsonized target cells. We also measured translocated microbial products in plasma and found differences in phylum distribution between HESN and HESC participants that were associated with the immune phenotypes affecting the risk of HIV-1 acquisition. Finally, a logistic regression model combining features of NK cell activation, α4β7 expression on memory CD4+ T cells, and T-box expressed in T cells (Tbet) expression by iNKT cells achieved the highest accuracy in identifying HESN and HESC participants. This immune signature, consisting of increased α4β7 on cells susceptible to HIV infection combined with higher NK cell activation and lower gut-homing potential, could affect the efficacy of HIV-1 prevention strategies such as vaccines.
Authors
Kawthar Machmach, Kombo F. N’guessan, Rohit Farmer, Sucheta Godbole, Dohoon Kim, Lauren McCormick, Noemia S. Lima, Amy R. Henry, Farida Laboune, Isabella Swafford, Sydney K. Mika, Bonnie M. Slike, Jeffrey R. Currier, Leigh Anne Eller, Julie A. Ake, Sandhya Vasan, Merlin L. Robb, Shelly J. Krebs, Daniel C. Douek, Dominic Paquin-Proulx, for the RV217 Study Group
-
Abstract
Cellular susceptibility to HIV is associated with integrin α4β7, a mucosal homing receptor involved with trafficking HIV target cells to sites of HIV replication. However, studies investigating preinfection α4β7 expression as a predictor of HIV outcomes have yielded inconsistent findings, raising questions about the role of α4β7 in HIV acquisition across populations. In this issue of the JCI, Machmach et al. assessed PBMCs collected before HIV infection and found higher α4β7 expression on memory CD4+ T cells and invariant NK T (iNKT) cells in individuals who went on to acquire HIV. Here, we consider possible explanations that may underlie discrepancies among studies and suggest that α4β7 should be considered as part of a multifactorial profile for determining HIV risk. While unlikely to serve as a target for HIV prevention or therapy, α4β7-directed interventions may offer adjunctive benefits in preserving or improving mucosal immunity.
-
T cells expressing the gut-homing integrin α4β7
The odds of HIV transmission are determined by both the infectiousness of the donor and the susceptibility of the recipient. While measuring HIV exposure is feasible in certain contexts such as serodiscordant couples, it remains difficult in many observational studies. Consequently, much of the research on HIV acquisition has focused on identifying biological and epidemiological factors that influence recipient susceptibility. Inflammation and its downstream effects, including recruitment of HIV target cells and compromised mucosal barrier integrity, are considered central to host vulnerability (1, 2). Among HIV’s primary targets are CD4+ T cells, which are heterogeneous in their ability to support infection and viral propagation (3). A subset of these cells expressing the gut-homing integrin α4β7 have received considerable attention for their potential role in HIV susceptibility and pathogenesis.
Although a role for α4β7 in HIV cure strategies remains limited (4–6), α4β7-expressing CD4+ T cells are consistently characterized as highly permissive to HIV. These cells are among the first to be depleted during acute infection (7), underscoring their preferential targeting. They often coexpress CCR5, the main HIV coreceptor, and markers of Th17 cells, another highly susceptible subset (7, 8). The inherent role of these T cells in trafficking cells to mucosal tissues through interaction with mucosal addressin cell adhesion molecule 1 (MAdCAM-1) enables their strategic localization at anatomical sites critical for HIV transmission and replication, maintaining a pool of target cells for infection during exposure (9). In vitro studies also suggest that some HIV strains can bind α4β7, not as an essential coreceptor, but as an auxiliary adhesion factor that facilitates virus attachment (10, 11). The relationship between preinfection α4β7 expression on memory CD4+ T cells and HIV acquisition risk has been investigated across multiple cohorts, with inconsistent findings. As such, α4β7 as a reliable predictor of HIV acquisition in humans remains contentious.
-
α4β7 as part of a composite immune signature
In this issue of the JCI, Machmach et al. (12) add evidence to the evolving literature on α4β7 and HIV susceptibility. Their study assessed α4β7 expression on PBMCs collected a median of 79 days prior to HIV infection in the RV217 study cohort, which consists of predominantly male and transgender individuals in Thailand at high risk of HIV exposure. The analysis compared 25 highly exposed seroconverters (HESCs) with 74 matched highly exposed seronegative (HESN) controls. Consistent with prior hypotheses, HESC individuals had higher α4β7 expression on memory CD4+ T cells and invariant NK T (iNKT) cells. These findings support the idea that elevated α4β7 expression may increase an individual’s susceptibility to HIV acquisition, particularly when expressed on cell subsets already highly permissive to HIV. Interestingly, the study also observed lower α4β7 expression on NK cells in HESC individuals, which suggests a reduced capacity for these cells to home to mucosal sites where early viral control is critical. Affected NK cells showed a decreased response to HIV-infected cells, as indicated by their diminished activity against cells coated with plasma from people with HIV, implying impairment in their antiviral function. Ultimately, α4β7 expression contributed to a composite immune signature, when combined with NK cell activation and iNKT transcriptional markers, that most effectively distinguished HESC from HESN participants. These results suggest that, while α4β7 may lack consistent predictive power on its own, its utility may lie in combination with other immune correlates as part of a multifactorial risk profile. Thus, α4β7 should be viewed not solely as a marker of cellular susceptibility, but as a component of a broader immunological landscape that collectively shapes the risk of HIV acquisition.
The promise of α4β7 as a biomarker of HIV risk was first raised in our earlier work, which showed that higher α4β7 expression on CD4+ T cells predicted both increased acquisition and accelerated disease progression among South African women in the CAPRISA 004 trial (13). However, follow-up studies have yielded divergent findings. For example, a multi-cohort study involving men who have sex with men (MSM), transgender individuals, and people who inject drugs (PWIDs) in the United States (HVTN 505 and ALIVE cohorts) found no association and a protective signal in PWIDs (14). In our more recent work examining preinfection PBMCs across African cohorts – HVTN 503 in South Africa and Partners PrEP and COS cohorts in East Africa — α4β7 was not a predictor of HIV acquisition (15). Rather, opposing trends emerged: in South African women especially (HVTN 503), α4β7 expression tended to correlate with greater risk, while in Ugandan participants, higher expression appeared to be protective. Only HVTN 503 showed an association with faster progression (15). These differences suggest region- or context-specific effects that complicate the interpretation of α4β7’s role.
The recent RV217 study extends this line of research by incorporating α4β7 expression into a multidimensional immune framework (Figure 1) (12). While measuring multiple susceptibility factors in parallel is ideal, Machmach and colleagues also shed more light on the specific HIV target cell hypothesis that has been raised for α4β7 based on a large body of evidence suggesting that α4β7-expressing cells have increased HIV susceptibility (16). Variability in findings between studies may reflect differences in cohort composition, viral clade, transmission route, age distribution, and other factors. For instance, HIV clades AE and C, which are predominant in Thailand and South Africa, respectively, have been shown to bind α4β7 more efficiently in vitro (13, 17), potentially explaining stronger associations in those regions. Likewise, sex-based biological differences in mucosal immunity and route of HIV transmission may affect host associations of HIV risk. Age-related changes in naive and memory T cell subsets and prior exposure history may modulate the relationship between α4β7 and HIV risk; it should be noted that virtually all α4β7+ T cells are in the memory compartment, and as individuals age, their naive:memory compartment contracts, affecting α4β7 expression levels. While age was matched and/or adjusted for in most studies, it is noteworthy that stronger α4β7 associations have often been observed in younger cohorts. Additionally, reliance on behavioral criteria to define high-risk groups introduces uncertainty in exposure levels, and small sample sizes may further limit statistical power. Another limitation is the lack of mucosal and tissue sampling in most studies of HIV susceptibility, precluding evaluation of immunological factors at sites of HIV exposure.
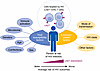 Figure 1
Figure 1Complex considerations of interacting biological and epidemiological factors that influence the rates of HIV transmission. Host and exposure factors may influence α4β7 frequencies and/or determine associations between α4β7 and HIV outcomes. Interpreting α4β7 in the context of multiple, interacting biological and epidemiological factors is critical when assessing HIV acquisition risk. A similar concept may apply to additional biological predictors or mediators of HIV clinical outcomes.
-
Clinical implications
Beyond its biological plausibility, interest in α4β7 stems from its therapeutic potential. Monoclonal antibodies targeting the α4β7 pathway are approved and considered first-line therapy for the treatment of inflammatory bowel disease (IBD) (18), prompting speculation about their potential use in HIV, where gastrointestinal (GI) disturbances have also been well documented. While it is unlikely these agents will be repurposed as standalone interventions for HIV prevention or cure, they may have adjunctive benefits, such as reducing gut inflammation and restoring mucosal immunity. Supporting this concept, a study in SIV-infected macaques receiving antiretroviral therapy found that combining anti-α4β7 antibodies with IL-21 enhanced immune responses, preserved mucosal T cells, and improved gut microbiome recovery (19). Although the therapy did not prevent viral rebound after antiretroviral therapy (ART) interruption, modest improvements in post-treatment viral control were observed, reinforcing interest in α4β7 as a potential immunomodulatory target. Studies have also suggested that anti-α4β7 monoclonal antibody reduces lymphoid aggregates in the GI tract, which could contribute to its influence on mucosal inflammation (20, 21). Whether anti-α4β7 monoclonal antibody effects in IBD can translate into improved gut health in people living with HIV remains to be seen.
As HIV incidence declines globally, identifying biological predictors of HIV acquisition in prospective cohort studies will become increasingly difficult, underscoring the need for meta-analyses that combine immunologic and exposure data across cohorts. In parallel, continued research into immune signatures of susceptibility and pathogenesis, such as those involving α4β7, can enhance our understanding of host-virus dynamics and help identify vulnerable populations who may benefit most from intensified intervention strategies. Importantly, the emergence of highly effective long-acting preexposure prophylaxis (PrEP) offers a powerful tool that can overcome heterogenous HIV risk that exists within populations (22). While such interventions and general declines in HIV incidence will hamper efforts to study natural HIV acquisition, ensuring equitable access to these biomedical interventions remains a public health imperative that can have a substantial effect on controlling the HIV pandemic.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Omole et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(15):e195258. https://doi.org/10.1172/JCI195258.
See the related article at NK cell activation and CD4+ T cell α4β7 expression are associated with susceptibility to HIV-1.
-
References
- Deeks SG, et al. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633–645.
- Sáez-Cirión A, Sereti I. Immunometabolism and HIV-1 pathogenesis: food for thought. Nat Rev Immunol. 2021;21(1):5–19.
- Tolomeo M, Cascio A. The complex dysregulations of CD4 T cell subtypes in HIV infection. Int J Mol Sci. 2024;25(14):7512.
- Byrareddy SN, et al. Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science. 2016;354(6309):197–202.
- Di Mascio M, et al. Evaluation of an antibody to α4β7 in the control of SIVmac239-nef-stop infection. Science. 2019;365(6457):1025–1029.
- Iwamoto N, et al. Blocking α4β7 integrin binding to SIV does not improve virologic control. Science. 2019;365(6457):1033–1036.
- Tokarev A, et al. Preferential infection of α4β7+ memory CD4+ T cells during early acute human immunodeficiency virus type 1 infection. Clin Infect Dis. 2020;71(11):e735–e743.
- Mijiti Z, et al. α4β7high CD4+ T cells are prone to be infected by HIV-1 and associated with HIV-1 disease progression. HIV Med. 2022;23 Suppl 1(s1):106–114.
- Liu Q, Lusso P. Integrin α4β7 in HIV-1 infection: a critical review. J Leukoc Biol. 2020;108(2):627–632.
- Richardson SI, et al. South African HIV-1 subtype C transmitted variants with a specific V2 motif show higher dependence on α4β7 for replication. Retrovirology. 2015;12:54.
- Goes LR, et al. The V2 loop of HIV gp120 delivers costimulatory signals to CD4+ T cells through Integrin α4β7 and promotes cellular activation and infection. Proc Natl Acad Sci U S A. 2020;117(51):32566–32573.
- Machmach K, et al. NK cell activation and CD4+ T cell α4β7 expression are associated with susceptibility to HIV-1. J Clin Invest. 2025;135(15):e187992.
- Sivro A, et al. Integrin α4β7 expression on peripheral blood CD4+ T cells predicts HIV acquisition and disease progression outcomes. Sci Transl Med. 2018;10(425):eaam6354.
- Martin AR, et al. The association of α4β7 expression with HIV acquisition and disease progression in people who inject drugs and men who have sex with men: case control studies. EBioMedicine. 2020;62:103102.
- Omole TE, et al. Pre-human immunodeficiency virus (HIV) α4β7hi CD4+ T cells and HIV risk among heterosexual individuals in Africa. J Infect Dis. 2025;231(4):e770–e780.
- Arthos J, et al. The Role of integrin α4β7 in HIV pathogenesis and treatment. Curr HIV/AIDS Rep. 2018;15(2):127–135.
- Peachman KK, et al. Identification of new regions in HIV-1 gp120 variable 2 and 3 loops that bind to α4β7 integrin receptor. PLoS One. 2015;10(12):e0143895.
- Cherry LN, et al. Vedolizumab: an α4β7 integrin antagonist for ulcerative colitis and Crohn’s disease. Ther Adv Chronic Dis. 2015;6(5):224–233.
- Johnson SD, et al. IL-21 and anti-α4β7 dual therapy during ART promotes immunological and microbiome responses in SIV-infected macaques. JCI Insight. 2025;10(6):e184491.
- Canales-Herrerias P, et al. Gut-associated lymphoid tissue attrition associates with response to anti-α4β7 therapy in ulcerative colitis. Sci Immunol. 2024;9(94):eadg7549.
- Uzzan M, et al. Anti-α4β7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1-infected individuals. Sci Transl Med. 2018;10(461):eaau4711.
- Abdool Karim Q. Enhancing HIV prevention with injectable preexposure prophylaxis. N Engl J Med. 2021;385(7):652–653.
-
Version history
- Version 1 (August 1, 2025): Electronic publication



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

