Advertisement
Review Series
Open Access |  10.1172/JCI194751
10.1172/JCI194751
Antiinflammatory actions of glucagon-like peptide-1–based therapies beyond metabolic benefits
Chi Kin Wong1 and Daniel J. Drucker1,2
1Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, Ontario, Canada.
2Department of Medicine, University of Toronto, Ontario, Canada.
Address correspondence to: Daniel J. Drucker, Lunenfeld-Tanenbaum Research Institute, Mt. Sinai Hospital, 600 University Avenue Mailbox 39 TCP 5-1004, Toronto, Ontario M5G 1X5, Canada. Phone: 416.361.2661; Email: drucker@lunenfeld.ca.
Find articles by
Wong, C.
in:
PubMed
|
Google Scholar
|

1Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, Ontario, Canada.
2Department of Medicine, University of Toronto, Ontario, Canada.
Address correspondence to: Daniel J. Drucker, Lunenfeld-Tanenbaum Research Institute, Mt. Sinai Hospital, 600 University Avenue Mailbox 39 TCP 5-1004, Toronto, Ontario M5G 1X5, Canada. Phone: 416.361.2661; Email: drucker@lunenfeld.ca.
Find articles by
Drucker, D.
in:
PubMed
|
Google Scholar
|

Published November 3, 2025 - More info
J Clin Invest. 2025;135(21):e194751. https://doi.org/10.1172/JCI194751.
© 2025 Wong et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Therapies based on glucagon-like peptide-1 (GLP-1) reduce rates of cardiovascular and chronic kidney disease in people with type 2 diabetes and/or obesity, with ongoing clinical trials investigating their effects in people with metabolic liver disease, arthritis, and both substance use and neurodegenerative disorders. Acute and chronic activation of GLP-1 receptor signaling also reduces systemic and tissue inflammation in mice and humans, through weight loss–dependent and –independent mechanisms, actions that may contribute to the expanding spectrum of clinical benefits ascribed to GLP-1 medicines. In this Review, we highlight current understanding of the direct and indirect antiinflammatory effects and mechanisms of GLP-1 medicines in both preclinical and clinical studies, covering emerging concepts, clinical relevance, and areas of uncertainty that require further investigation.
The development of medicines based solely or in part on glucagon-like peptide-1 (GLP-1) action, herein referred to as GLP-1 medicines, has transformed the treatment options for people with type 2 diabetes (T2D) who are unable to achieve sufficient glucose control with existing therapies. GLP-1 medicines have also produced meaningful benefits in individuals living with obesity who are unable to achieve sufficient weight loss through lifestyle changes and who are not eligible for bariatric surgery. Modern GLP-1 medicines such as semaglutide and tirzepatide frequently result in more than 10% weight loss in people living with obesity, while maintaining a favorable safety profile. Outcome studies show that GLP-1 medicines reduce rates of complications associated with T2D and/or obesity, including cardiovascular diseases, metabolic dysfunction–associated liver diseases, chronic kidney disease, osteoarthritis, and obstructive sleep apnea (Figure 1). While the metabolic benefits of GLP-1 medicines, such as improved glucose control and weight loss, improve health and likely help reduce metabolic disease–associated complications, a growing body of evidence suggests that GLP-1 has intrinsic antiinflammatory actions that are independent of its metabolic actions.
 Figure 1
Figure 1Antiinflammatory actions of GLP-1 medicines across organs. GLP-1R agonists exert broad antiinflammatory effects in multiple peripheral organs, including the central nervous system, lungs, cardiovascular system, liver, intestine, kidneys, and joints. Evidence from both preclinical and clinical studies supports their antiinflammatory roles in the cardiovascular system, liver, kidneys, and joints. However, the potential antiinflammatory effects of GLP-1 signaling in the central nervous system, lungs, and intestine remain to be fully elucidated and warrant further investigation.
Earlier generations of GLP-1 medicines such as exenatide, liraglutide, dulaglutide, and semaglutide function solely as GLP-1 receptor (GLP-1R) agonists. Recently, clinical trials of tirzepatide, a dual GLP-1R and glucose-dependent insulinotropic polypeptide receptor (GIPR) agonist, have achieved approximately 20% weight loss in substantial proportions of people with obesity (1). While the GIPR agonism of tirzepatide likely augments the weight loss effect of GLP-1R activation, GIPR signaling may also contribute to antiinflammatory responses through direct actions on GIPR-expressing immune cells. In addition, several emerging GLP-1 medicines simultaneously target receptors for hormones such as GIP, glucagon, and amylin and are currently being evaluated for the treatment of obesity and related metabolic conditions (2–4). Understanding how GLP-1 regulates inflammation (Figure 1) provides insights into the pleiotropic weight loss–independent benefits of GLP-1R agonism, potentially explaining its actions to reduce the severity of several chronic diseases marked by dysregulated immune function and inflammation.
-
GLP-1–producing L cells as pathogen and inflammation sensors
L cells respond to nutrients, bile acids, microbial metabolites, and proinflammatory molecules that trigger GLP-1 release (Figure 2). Administration of LPS, an endotoxin derived from Gram-negative bacteria that activates innate immunity and increases proinflammatory cytokines such as TNF-α, IL-6, and IL-1β, elevates circulating GLP-1 within 3 hours in mice and humans (5–7). Similarly, IL-1β or IL-6 injection increases GLP-1 levels in mice within the same time frame (5). Both LPS- and IL-1β–induced GLP-1 release partly depends on IL-6 as shown in Il6–/– mice (5, 6), although the source of IL-6 that triggers L cells is unclear. Plasma GLP-1 is also elevated in mice with colitis induced by adoptive transfer of CD4+CD25– T cells (8), suggesting that a compromised intestinal barrier and translocation of intestinal microbes promote GLP-1 release. L cells sense LPS via TLR4 on their basolateral membrane (6), enabling GLP-1 secretion during local and systemic inflammation arising from intestinal barrier dysfunction.
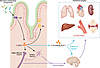 Figure 2
Figure 2The endogenous intestinal GLP-1 circuit as a pathogen and inflammation sensor. TLR4 expression on the basolateral surface of enteroendocrine L cells enables them to respond to endotoxins and endogenous inflammatory signals, triggering the release of GLP-1. Within the intestine, locally secreted GLP-1 can act in a paracrine manner on nearby GLP-1R–expressing cells, including IELs, enteric neurons, and vagal afferent neurons. GLP-1 is also released into the portal system and enters the systemic bloodstream, where it functions as a classical endocrine hormone, reaching peripheral organs to modulate inflammation. Alternatively, signals initiated in the intestine may be transmitted through the intestinal neural network, enabling gut-brain communication that indirectly monitors and influences systemic inflammatory processes.
In humans, LPS infusion raises GLP-1 levels in healthy young subjects (9). Circulating GLP-1 levels also correlate with severity of critical illnesses (5, 10), sepsis (11), end-stage kidney disease (11), respiratory failure (12), and intestinal ischemia-reperfusion injury (6). However, unlike in mice, acute IL-6 infusion up to 15 μg does not increase GLP-1 levels in humans (13), suggesting species differences in GLP-1 regulation by inflammation.
The role of endogenous GLP-1 in inflammation remains unclear. In addition to L cells, preproglucagon neurons in the brain stem produce GLP-1, but how inflammation affects these neurons, and the role of brain-derived GLP-1 in local or systemic inflammation, are unknown. This is particularly relevant given the role of the brain stem in regulating the vagal pathway and the cholinergic antiinflammatory reflex (14), suggesting a possible yet under-investigated neuroimmune link. The effects of impaired GLP-1 secretion or reduced endogenous levels on inflammation have also not been fully explored. Furthermore, while acute inflammation rapidly elevates GLP-1 levels, it remains uncertain whether these increases persist during chronic inflammation. Notably, evidence from whole-body Glp1r knockout mice, discussed elsewhere in this Review, suggests that endogenous GLP-1R signaling plays a critical role in modulating inflammation, highlighting the need for further investigation.
-
Weight loss–independent antiinflammatory effects of GLP-1 medicines
Systemic inflammation can result from local tissue inflammation or widespread immune activation. While proinflammatory signals play key roles in host defense and tissue repair, sustained low-grade inflammation can drive organ dysfunction and the development of chronic diseases. C-reactive protein (CRP), an acute-phase protein, serves as a surrogate marker of systemic inflammation in humans. Multiple GLP-1 medicines, including exenatide (15), liraglutide (16), semaglutide (17, 18), and tirzepatide (19), reduce plasma CRP levels in people living with T2D and/or obesity. These effects are likely mediated in part by improvements in glucose control and weight loss. More potent peptides such as liraglutide, semaglutide, and tirzepatide may have greater antiinflammatory effects, consistent with correlations between CRP and weight loss seen in lifestyle interventions and bariatric surgery (20).
However, GLP-1 medicines also exert antiinflammatory effects independent of their metabolic benefits. For example, a single dose of exenatide or semaglutide reduced circulating TNF-α levels in mice challenged with LPS (21, 22). Separately, exenatide inhibited binding of NF-κB to DNA and downregulated TNF and IL1B in human PBMCs (23). These acute effects occur within hours and precede any meaningful weight loss.
Clinical trial data further support weight loss–independent antiinflammatory effects of GLP-1 medicines. In the semaglutide SUSTAIN and PIONEER trials, reductions in glucose and weight explained only 20%–60% of the observed CRP reductions (17). In PIONEER 2, oral semaglutide reduced CRP by 30%, whereas empagliflozin had no effect despite similar approximately 4% weight loss (24). Proteomic analyses from the STEP weight loss trials in people with or without T2D revealed semaglutide-induced changes in inflammatory and immune regulatory pathways that could not be fully attributed to metabolic improvements alone (25). Hence, preclinical and clinical studies of GLP-1 medicines, administered either acutely or chronically, suggest that a significant portion of the antiinflammatory effects of GLP-1 medicines is mediated by mechanisms independent of metabolic changes.
The balance between weight loss–dependent and –independent antiinflammatory effects of GLP-1 medicines likely varies across disease contexts, depending on the underlying drivers of inflammation. Understanding GLP-1–based therapy mechanisms will require considering systemic versus local effects and distinguishing between immune and non-immune pathways. Although GLP-1R expression in peripheral tissues is generally low and restricted to rare cell types (26), antiinflammatory benefits have been observed in various preclinical models, such as obesity, atherosclerosis, steatohepatitis, and nephritis, even in the absence of direct immune cell targeting (27–32). These findings suggest that GLP-1 medicines modulate inflammation through multiple pathways, including systemic metabolic improvements, direct actions on immune cells, and effects on non-immune cells that support interorgan communication of antiinflammatory signals (Figure 1 and Figure 2).
-
GLP-1R expression in the immune system
GLP-1 medicines regulate inflammation partly by directly modulating immune cells, although GLP-1R expression is typically low in lymphoid organs (Figure 1) (26). Thymus, bone marrow, spleen, mesenteric lymph nodes, brachial/axillary lymph nodes, inguinal lymph nodes, and PBMCs express low levels of Glp1r (29, 33, 34). Lymphoid cells express Glp1r more often than myeloid cells (35), with tissue-resident lymphocyte subsets showing stronger expression. In the small intestine, intraepithelial lymphocytes (IELs) express high levels of Glp1r, triggering cAMP response upon activation by exendin-4 (33). In mice lacking Glp1r in the Lck expression domain (Glp1rLck–/–), Glp1r expression is abolished in multiple IEL subsets, including CD8αα+TCRαβ+ and CD8αβ+TCRαβ+ IELs, with an approximately 50% reduction observed in CD8αα+TCRγδ+ IELs (34). Glp1rLck–/– mice also display Glp1r downregulation in the thymus and mesenteric lymph nodes, implicating T cell–derived Glp1r in IEL development. In the liver, γδ T cells and some CD8+ T cells express full-length Glp1r transcripts (29). In the spleen, some CD4+ and CD8+ T cells show GLP-1R immunoreactivity, although transcript levels are less than one-thousandth of those in mouse islets (36).
Glp1r expression in myeloid cells is very low compared with that in lymphocytes. Glp1rLck–/– mice retain normal Glp1r levels in spleen and PBMCs (34), whereas mice with GLP-1R deletion in the Tie2 expression domain (Glp1rTie2–/–) show complete loss of Glp1r in these compartments (29), suggesting expression in non–T cell hematopoietic or endothelial cells. Glp1r expression is undetectable by quantitative PCR in peritoneal or adipose tissue macrophages (37), although Lyz2-Cre–driven deletion of Glp1r (Glp1rLyz2–/–) abolishes expression in bone marrow–derived macrophages (38). Notably, Glp1rTie2–/– mice do not show reduced Glp1r in the bone marrow (29). Consistent with the low GLP-1R signal in the PBMCs and splenocytes, treatment of mouse whole blood or splenocyte cultures with 50 nM exenatide did not alter LPS-induced TNF-α release (22). However, this does not exclude the possibility that GLP-1R becomes upregulated or responsive in disease, or that innate immunity is affected indirectly.
Detecting GLP-1R on immune cells remains technically difficult. Flow cytometry permits co-detection of GLP-1R with immune markers, but the reliance on antibodies underscores the importance of rigorous reagent validation. The limited specificity of many antibodies against mouse and human GLP-1R, combined with the challenges of detecting the receptor at low abundance, has been well documented (37, 39, 40). A widely used anti–GLP-1R antibody fails to reliably detect the receptor in T cells, producing signals even in Glp1r–/– T cells (41). Therefore, accurate detection of GLP-1R requires combined approaches, including full-length Glp1r/GLP1R cDNA detection, in situ hybridization, and antibody-based methods validated with knockout controls.
GLP-1 medicines suppress specific subsets of T cell activity through GLP-1R. A single injection of 42 μg/kg exenatide reduced circulating levels of IFN-γ, TNF-α, and IL-2 in mice challenged with agonistic anti-CD3 antibodies, which broadly activate T cells and provokes systemic and gut inflammation (34). Gut IEL GLP-1R mediates local antiinflammatory actions in the small intestine by inhibiting proximal T cell receptor (TCR) signaling in a protein kinase A–dependent manner, reducing phosphorylation of ZAP70 and SLP-76, which are key signaling nodes for initiating TCR-driven cytokine expression (Figure 3). This signaling inhibition dampens T cell effector functions, including cytotoxicity and the production and secretion of IFN-γ, TNF-α, and IL-2. In another study, in vivo treatment with 70 μg/kg exenatide twice daily for 7 days improved survival of BALB/c islet or heart allografts in C57BL/6J mice (36). These phenotypes parallel in vitro findings that 10 nM exenatide treatment reduces IFN-γ production in splenic T cells. While these findings implicate T cell–intrinsic GLP-1R signaling, definitive attribution awaits studies employing T cell–specific GLP-1R knockout models. Notably, in a mismatched graft-versus-host disease (GvHD) model, C57BL/6J recipients of donor BALB/c T cells developed small-intestinal inflammation, and donor T cells within the small-intestinal epithelium expressed functional GLP-1R (42). Yet 10 μg/kg semaglutide treatment throughout disease induction failed to blunt this pathology. Moreover, inducing GvHD by giving GLP-1R–knockout T cells to BALB/c recipients did not exacerbate the disease. Considered alongside the host-versus-graft allograft protection (36), these data suggest that GLP-1–mediated immunomodulation may act mainly through the host compartment under some but not all inflammatory conditions.
 Figure 3
Figure 3Inhibitory effects of GLP-1 medicines on immune cell function. GLP-1 medicines generally exert inhibitory effects on immune cell function. Across multiple T cell populations, they suppress the secretion of interferon-γ (IFN-γ). In intraepithelial lymphocytes (IELs) and invariant natural killer T (iNKT) cells, GLP-1 medicines also induce cyclic adenosine monophosphate (cAMP) and cAMP-responsive element–binding protein (CREB), indicating engagement of the cAMP/protein kinase A signaling pathway. In IELs specifically, the activation of GLP-1R signaling suppresses phosphorylation of ζ chain–associated protein kinase 70 (ZAP70) and its downstream target SH2 domain–containing leukocyte protein 76 (SLP-76), leading to dampening of proximal T cell receptor signaling and reduced cytokine transcription. This mechanism may be shared by iNKT and other T cells expressing the GLP-1R. In myeloid cells, GLP-1 medicines also attenuate proinflammatory cytokine release, although these effects appear to be primarily indirect.
In humans, GLP-1R immunoreactivity has been detected in T cells by flow cytometry, including in invariant natural killer T (iNKT) cells (43), PBMC T cells (36), and chimeric antigen receptor (CAR) T cells (44). Human iNKT cells treated with 3.5 nM liraglutide show increased cAMP within 30 minutes, and 18 nM liraglutide inhibits IFN-γ and IL-4 secretion upon α-galactosylceramide stimulation presented by CD1d-expressing cells (43). Alongside similar inhibitory effects on mouse splenic T cells (36), these results align with the dampening of TCR signaling by GLP-1R activation observed in mouse gut IELs (34).
-
Indirect actions of GLP-1 medicines on immune function
In addition to directly acting on immune cells, GLP-1 medicines acutely attenuate systemic inflammation through central mechanisms. In mice, exenatide and semaglutide reduce plasma TNF-α levels induced by agonists for TLR1, TLR2, TLR4, TLR5, and TLR9 or by cecal slurry–induced polymicrobial sepsis (22). This effect requires GLP-1R expression in brain neurons, as shown in mice lacking GLP-1R in neuron-targeting Wnt1- or nestin-expressing domains (Glp1rWnt1–/– and Glp1rNes–/–), and by intracerebroventricular injection of exendin-9-39, a GLP-1R antagonist that suppresses activation of brain GLP-1R signaling. Furthermore, the antiinflammatory action also depends on intact α1-adrenergic and δ-opioid receptor signaling, as shown by pharmacological blockade of these receptors, implicating neuro-immune crosstalk in GLP-1–mediated suppression of peripheral inflammation. Whether this neural mechanism explains improved circulating inflammatory biomarkers in people treated with GLP-1 medicines is challenging to ascertain (25).
-
GLP-1 medicines in neuroinflammation
Neuroinflammation is a hallmark of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases (45). Microglia, the primary immune responders in the brain, release proinflammatory cytokines when activated, which then stimulate nearby reactive astrocytes, amplifying local neuroinflammation. Twice-weekly treatment with 3 mg/kg of the PEGylated exenatide NLY01 for 5 months reduced IL-1α, TNF-α, and C1q secretion by primary mouse microglia exposed to α-synuclein preformed fibrils, thereby limiting astrocyte conversion to the neurotoxic A1 phenotype (46). A 1-hour pretreatment with 1 μM NLY01 blocked nuclear translocation of NF-κB in LPS-stimulated primary mouse microglia (47). It remains unclear whether these neural antiinflammatory actions of GLP-1 medicines require microglial GLP-1R activity. Within the brain, GLP-1R is primarily expressed in neurons, and activation of neuronal GLP-1R signaling has been linked to systemic antiinflammatory effects (22). This raises the possibility that GLP-1 medicines may reduce neuroinflammation both directly, through actions on neurons, and indirectly, by lowering systemic inflammation that contributes to neuroinflammatory processes. Additionally, mouse astrocytes express GLP-1R (48). Microbiota depletion in HFD-fed mice elevates plasma GLP-1 levels and attenuates hypothalamic inflammation, actions that require the astrocyte GLP-1R (49). The potential actions of GLP-1 medicines in modulating astrocyte-mediated neuroinflammation have not been extensively explored.
In mouse models of Parkinson’s disease induced by either α-synuclein preformed fibrils or α-synuclein overexpression, treatment with 3 mg/kg NLY01 twice weekly for 5 months protected against neurodegeneration, including α-synuclein aggregation and loss of dopaminergic neurons (46). In 7-month-old APP/PS1 mice, a transgenic model of Alzheimer’s disease that expresses the human version of the amyloid polypeptide and a mutant presenilin-1, two months of daily 100 μg/kg liraglutide treatment improved memory function as measured by object recognition and Morris water maze tests, reduced amyloid plaque load, and decreased the frequency of the microglial activation marker Iba-1 (50). Yet 6 to 7 weeks of daily treatment with semaglutide (escalated from 41 μg/kg to 103 μg/kg) or tirzepatide (escalated from 12 μg/kg to 48 μg/kg) did not produce any noticeable effect on these readouts, including Iba-1 activation, in 12-month-old 5XFAD and APP/PS1 mice (51). Many of these neuroprotective effects of GLP-1 medicines in preclinical studies remain to be causally associated with the antiinflammatory actions of GLP-1 medicines (52).
Multiple clinical trials assessing the therapeutic potential of GLP-1 medicines for Parkinson’s disease have been completed. Treatment with exenatide or lixisenatide for 1 year produced a modest but positive effect on Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III scores in people with recently diagnosed Parkinson’s disease (15, 53). In contrast, NLY01, despite being designed for greater brain penetration, showed no benefit for MDS-UPDRS part III scores (54). A phase III trial evaluating 96 weeks of once-weekly exenatide treatment in people with Parkinson’s disease also found no disease-modifying benefits for MDS-UPDRS part III scores (55). Ongoing phase III trials of semaglutide for Alzheimer’s disease (EVOKE/EVOKE+) will offer further insights into whether newer GLP-1 medicines may protect against Alzheimer’s disease. While these benefits for neurodegeneration may involve antiinflammatory actions, direct attribution to such effects remains to be conclusively demonstrated.
-
GLP-1 medicines in cardiovascular inflammation
Weight loss, blood pressure reduction, reduction in atherogenic lipid profiles, and antiinflammatory effects all contribute to the cardiovascular protection conferred by GLP-1 medicines. These mechanisms work synergistically to reduce atherosclerosis, a proinflammatory process that drives the progression of cardiovascular diseases, such as coronary heart disease, peripheral vascular disease, and stroke. Although Glp1r expression is mostly localized to endothelial cells in the mouse aorta (29), other vascular cell types may express Glp1r under atherogenic conditions, making it difficult to pinpoint which cell type mediates the antiinflammatory effects of GLP-1 medicines. Treatment with 1 mg/kg liraglutide for 14 weeks reduced atherosclerotic plaque size in the aorta of Apoe–/– mice (21). Control mice with weight loss matching that of mice receiving 1 mg/kg liraglutide were not protected from atherosclerosis, suggesting that liraglutide has weight loss–independent anti-atherogenic effects. Treatment with semaglutide at a low dose of 4 μg/kg for 12 to 17 weeks reduces plaque size and downregulates a myeloid proinflammatory gene program in Apoe–/– and Ldlr–/– mice. Similarly, 10 μg/kg semaglutide treatment for 18 weeks reduced aortic plaque burden in mice with atherosclerosis induced by Pcsk9 overexpression (29). This protective effect persisted in Glp1rTie2–/– mice, suggesting that the expression of immune cell or endothelial cell GLP-1Rs is not essential for this action. In a mouse model of angiotensin II–induced hypertension and cardiac hypertrophy, 30 μg/kg liraglutide treatment twice daily for 7 days reduced Ly6B.2 staining in the aorta and decreased the frequency of Ly6C+Ly6G– monocytes and Ly6C+Ly6G+ neutrophils (38). These immunomodulatory effects were abolished in mice lacking GLP-1R in the Cdh5 domain, which targets the endothelial lineage, but remained intact in myeloid-targeting Glp1rLyz2–/– mice. Notably, Cdh5-Cre can also be active in immune cells (56), suggesting that this model is not sufficient to rule out the involvement of non-myeloid immune cells.
In addition to the inhibitory effects on atherosclerosis, direct and indirect antiinflammatory actions of GLP-1 medicines on the heart may also contribute to their cardioprotective actions (Figure 3 and Figure 4. In a mouse model of acute myocardial infarction induced by coronary artery ligation, treatment with liraglutide for 7 days reduced atrial Il6 expression and circulating IL-6 levels 3 hours after the procedure (57). Liraglutide reduced the severity of acute myocardial infarction in control but not in Glp1rTie2–/– mice. In the transverse aortic constriction model, which causes pressure overload, ventricular compensation, and heart failure, studies have reported improvements with GLP-1 medicines in ventricular hypertrophy associated with reduced inflammation in rodents (58, 59), although none of these studies addresses the potential site(s) of GLP-1 action. As is postulated for atherosclerosis, GLP-1R–expressing endothelial cells and/or immune cells may contribute in part to the antiinflammatory actions in both acute and semi-chronic cardiac injury.
Cardiovascular outcome trials show that treatment with long-acting GLP-1 medicines such as liraglutide, dulaglutide, and semaglutide reduces cardiovascular events in people living with T2D and with semaglutide also in people living with obesity (60–62). These benefits appear quickly after initiation of treatment with semaglutide in people with overweight or obesity and a history of atherosclerotic heart disease, suggesting that GLP-1 medicines may reduce cardiovascular events in part through weight loss–independent mechanisms (62). Treatment with liraglutide or semaglutide lowers CRP levels, which correlates with the observed reduction in cardiovascular events in these trials. The efficacy of GLP-1 medications is consistent with actions of other antiinflammatory agents, such as IL-1 antagonists and colchicine, in reducing cardiovascular events (63, 64).
Both semaglutide and tirzepatide reduce the severity of heart failure with preserved ejection fraction (65, 66). The contribution of the antiinflammatory effects of GLP-1 medicines to this outcome is not clear. Reconciling the mechanisms of actions of GLP-1 medicines in mouse and human hearts is challenging because of the distinct patterns of GLP-1R expression. Both mouse and human endocardial endothelial cells express Glp1r/GLP1R (57). However, while Glp1r is not expressed appreciably in mouse cardiomyocytes, some human ventricular and atrial cardiomyocytes do express GLP1R (40, 57). This is further supported by the near-complete absence of cardiac Glp1r mRNA in Glp1rTie2–/– mice (57), suggesting that mouse cardiac Glp1r expression is primarily contributed by endothelial cells and/or immune cells. Thus, while endocardial endothelial cells represent a plausible target for the antiinflammatory actions of GLP-1 medicines locally in both species, these medicines may also exert cardioprotective effects directly on human cardiomyocytes.
-
GLP-1 medicines in liver inflammation
GLP-1 medicines are being investigated for the treatment of metabolic dysfunction–associated steatohepatitis (MASH), a metabolic liver disease with limited therapeutic options. Early preclinical studies focused on mice that develop diet-induced liver inflammation alongside atherosclerosis. Treatment with 30 μg/kg AC3174, an exenatide analog, administered via an osmotic pump for 28 days in Lepob mice on a high–trans-fat diet significantly reduced liver weight, hepatic lipid accumulation, and plasma alanine transaminase levels in comparison with pair-fed, weight-matched controls (30). However, no significant changes were observed in hepatic galectin-3 immunohistochemistry or Cd68 and Emr1 gene expression. These benefits were abolished in whole-body Glp1r-null (Glp1r–/–) mice on the same diet. In APOE*3-Leiden.CETP mice fed a cholesterol-containing high-fat diet that promotes atherosclerosis, 4 weeks of daily 50 μg/kg exenatide treatment protected against hepatic inflammation, as indicated by a decrease in CD68+, F4/80+, and CD18+/CD11b+ staining, as well as reduced Tnf, Il1b, and Il6 mRNA levels in the liver (67). In Ldlr–/– mice on a 40% high-fat diet for 17 weeks, daily administration of semaglutide at 4, 12, or 60 μg/kg downregulated collagen genes and immune-related genes including S100a8, S100a9, Timp1, Tlr2, and Cx3cr1 (21). Similarly, control mice with Pcsk9 overexpression–induced atherosclerosis treated with 10 μg/kg semaglutide for 18 weeks also showed reduced liver inflammation, as confirmed by quantitative PCR analysis of Col1a1, Tnf, Ccl2, Tgfb1, Cd3g, Il2, and Il4 expression (29). Notably, these effects were lost in Glp1rTie2–/– mice. However, these mice retained the inhibitory effects of semaglutide on atherosclerosis, suggesting that the improvements in liver inflammation occur independently of improvements in vascular inflammation via distinct cellular targets.
GLP-1 medicines induce substantial metabolic improvements in the liver, with reduction in liver weight and lipid accumulation largely attributed to weight loss. The relative contribution of metabolic versus direct antiinflammatory effects of GLP-1 medicines to reduce liver inflammation remains to be clarified. Both hepatic endothelial and T cells express the GLP-1R (29), suggesting that GLP-1 medicines may also act locally in the liver to reduce inflammation. Expanding the therapeutic actions of GLP-1 medicines from liver inflammation to prevention or even reversal of fibrosis in the absence of atherosclerosis would provide valuable mechanistic insights into how GLP-1 medicines improve MASH.
Clinical trials evaluating the effects of GLP-1 medicines in people living with MASH have reported favorable outcomes. Part 1 of a phase III trial investigating semaglutide for the treatment of MASH demonstrated resolution of steatohepatitis with no worsening of fibrosis in 63% of the semaglutide group, compared with 34% in the placebo group (68). In addition, 37% of the semaglutide group showed improvement in fibrosis without worsening of steatohepatitis, compared with 23% in the placebo group. Phase II trials for tirzepatide and survodutide, a GLP-1/glucagon receptor co-agonist, have also shown positive outcomes in people living with MASH and obesity, demonstrating reduction of inflammation without worsening of fibrosis (4, 69).
-
GLP-1 medicines in kidney inflammation
The glucose lowering, hemodynamic benefits, and antiinflammatory actions of GLP-1 medicines may partly explain their efficacy in people with T2D and chronic kidney disease (CKD). Experimental mouse models for both nephritis and CKD have been used to study the effects of GLP-1 medicines on kidney inflammation. Glp1r–/– mice with nephrotoxic serum–induced nephritis showed enhanced renal infiltration of neutrophils and CD4+ T cells, along with increased proinflammatory cytokine gene expression in the spleen and lymph nodes (31). Daily treatment with 200 μg/kg liraglutide for 2 weeks reduced albumin-to-creatinine ratios and decreased renal immune cell filtration in control but not in Glp1r–/– mice. In a diabetes-driven kidney disease model, Glp1r–/– mice with streptozotocin-induced diabetes exhibited spontaneous kidney dysfunction and accelerated kidney damage as evidenced by their higher albumin-to-creatinine ratios, increased fibronectin levels, and lower cystatin C levels in the urine (70). These changes were associated with elevated circulating levels of advanced glycation end products (AGEs), with kidney dysfunction partially mitigated by deletion of the receptor for AGEs (Ager) in Glp1r–/– mice. Thus, the absence of GLP-1R is associated with enhanced AGE production, which contributes to kidney inflammation and damage. In parallel, daily treatment with 50 μg/kg liraglutide for 20 weeks improved urine chemistry and podocyte histology in Ins2Akita mice, a transgenic model of spontaneous diabetes. The kidneys of liraglutide-treated mice also showed reduced DNA binding ability of NF-κB, decreased MCP-1 levels, and increased IL-10 levels. In a rat model of subtotal nephrectomy, which mimics CKD, 7 weeks of twice-daily liraglutide treatment attenuates Ager expression in the kidney. While these antiinflammatory effects have not been attributed to GLP-1 action in specific organs or cell types, the kidney GLP-1R has been localized primarily to vascular smooth muscle cells within renal arteries and arterioles using either 125I-labeled exendin-4, a validated anti–GLP-1R antibody, or in situ hybridization (71, 72). Whether the renal arterial GLP-1R mediates the antiinflammatory actions of GLP-1 medicines in the kidney remains to be determined.
As a secondary outcome of the AWARD-7 trial, which evaluated the effects of dulaglutide treatment in people with T2D and CKD, one year of treatment with dulaglutide resulted in a higher estimated glomerular filtration rate compared with insulin glargine (73). A follow-up post hoc analysis identified improvements in fibrosis-related biomarkers in the dulaglutide group, including lower serum PRO-C6 levels for type VI collagen formation and higher urine C3M levels for type III collagen degradation (74). These changes are consistent with potential improvements in kidney fibrosis. The FLOW trial was the first clinical trial that evaluated the effects of a GLP-1 medicine, semaglutide, on a composite outcome of progression of CKD and cardiovascular death, in people with T2D. Once-weekly treatment with 1 mg semaglutide reduced the risk of the primary composite outcome by 24% and delayed the progression of kidney dysfunction over a period of 4 years (75). Similar to findings from MASH clinical trials with semaglutide, the potential actions of GLP-1 medicines to delay or attenuate inflammation-associated fibrosis suggest that these drugs may inhibit the pathogenic proinflammatory cascade and subsequent fibrotic processes in multiple organs, either directly or indirectly.
-
GLP-1 medicines in pulmonary inflammation
GLP-1 medicines generally exhibit antiinflammatory effects in preclinical models of lung inflammation. A single 800 μg/kg dose of liraglutide administered 2 hours before LPS exposure attenuated lung injury and reduced lung Tnf, Il6, and Il1b expression in control but not in Glp1r–/– mice (76). Similarly, daily treatment with 200 μg/kg liraglutide downregulated Il6 and Ccl2 in the lungs of mice during the first 3 days after influenza infection (77). In a model of polymicrobial sepsis–induced lung inflammation, a single 10 μg/kg dose of semaglutide treatment suppressed lung Tnf, Il6, and Il1b expression and attenuated lung neutrophil elastase staining 24 hours after infection, an effect absent in Glp1rWnt1–/– mice with inactivation of neuronal GLP-1Rs (22). However, the response to GLP-1 medicines differs in a bleomycin-induced model of lung injury. In this model, Glp1r–/– mice exhibited reduced weight loss and lowered lung Il6, Il1b, Ccl2, and Cxcl2 expression, although survival remained unchanged (77). Notably, a 1-week treatment with 200 μg/kg liraglutide exacerbated bleomycin-induced lung injury in control but not Glp1r–/– mice, an effect prevented by cotreatment with atipamezole, an α2-adrenergic receptor antagonist. The contrasting effects observed in different lung injury models may stem from differences in immune activation: bleomycin-induced lung injury primarily triggers inflammation secondary to DNA damage, whereas LPS and pathogens directly activate innate and adaptive immune responses.
The specific GLP-1R–expressing cells modulating lung immune and inflammatory processes remain unclear. In situ hybridization has identified Glp1r expression in type 2 alveolar epithelial cells, which may contribute to Il6 and Ccl2 induction following bleomycin exposure (77). However, the majority of lung Glp1r expression appears to originate from endothelial and/or immune cells, as Glp1rTie2–/– and Glp1rCdh5–/– mice exhibit a >99% and >90% loss of lung Glp1r expression, respectively (29, 38). The precise localization of GLP-1R expression in human lungs remains unclear (78). However, post hoc analysis of the SELECT trial suggests that semaglutide use was associated with a reduced incidence of serious adverse events and mortality from COVID-19 (79), indicating potential clinical relevance for the antiinflammatory actions of GLP-1 medicines in lung inflammation and infection (Figure 3).
GLP-1 medicines are also efficacious in obstructive sleep apnea (OSA), a respiratory disorder that contributes to chronic inflammation and increases the risk of cardiometabolic diseases. A 32-week treatment with liraglutide reduced the apnea-hypopnea index by 12%, compared with 6% in the placebo group, among people with obesity and moderate to severe OSA (80). The SURMOUNT-OSA trial reported that tirzepatide reduced the apnea-hypopnea index by 25% to 29% over 52 weeks in individuals with obesity and moderate to severe OSA, compared with a reduction of 5.3% to 5.5% in the placebo group, regardless of the use of positive airway pressure therapy (19). This improvement was accompanied by a significant reduction in high-sensitivity CRP concentration, suggesting that the antiinflammatory effects of GLP-1 medicines may contribute to or be independently associated with their benefits in OSA.
-
GLP-1 medicines in intestinal inflammation
The intestine is a primary site of endogenous GLP-1 production (81), and likely responds to high local GLP-1 signaling in a paracrine manner, acting as both a sensor and a modulator of intestinal inflammation. A single injection of 42 μg/kg exenatide in anti-CD3–challenged mice downregulated Ifng in small-bowel IELs and suppressed interferon-stimulated genes, including Eif2ak2 and Usp18, in intestinal epithelial cells (34). These effects correlated with reduced crypt cell apoptosis, as indicated by decreased cleaved caspase-3 staining. The antiinflammatory and anti-apoptotic effects of exenatide were absent in Glp1rLck–/– mice, suggesting that GLP-1R signaling in gut IELs plays a critical role in modulating inflammation in the small intestine. The impact of GLP-1 medications on the gut microbiota, which is partly mediated by gut IELs, may also affect the intestinal response to inflammation and its interaction with the host immune system, warranting further investigation.
Glp1r–/– mice exhibited exacerbated intestinal inflammation in a dextran sulfate sodium–induced model of colitis (33). Similarly, in a colitis model induced by adoptive transfer of CD4+CD25– T cells, daily 600 μg/kg liraglutide treatment for 35 days after transfer significantly reduced the colon weight-to-length ratio and improved colonic histopathology, preserving colonic crypt structure and reducing leukocyte infiltration (8). Notably, GLP-1R expression in gut IELs is significantly lower in the colon than in the small intestine (33), suggesting that other cell types, such as enteric neurons (82), may contribute to the antiinflammatory actions of GLP-1 medicines in the colon.
Brunner’s glands, located at the proximal end of the duodenum, also play a role in GLP-1–mediated intestinal response to inflammation. A single dose of 1 mg/kg exenatide or 500 μg/kg liraglutide induced Il33, Muc5b, and Ccl20 expression in Brunner’s glands of control mice but not in Glp1r–/– mice (8). Interestingly, in colonic mucosal biopsies from individuals with inflammatory bowel disease, these genes were upregulated in inflamed tissue compared with noninflamed mucosa from the same patients, while GLP1R expression was downregulated.
Retrospective cohort studies suggest that the use of GLP-1 medicines is associated with improved inflammatory bowel disease outcomes in people with T2D (83, 84). Given that obesity is a common comorbidity of inflammatory bowel disease (85), further research is needed to understand the significance of the GLP-1R downregulation during inflammatory bowel disease and how GLP-1 medicines interact with inflammatory bowel disease through both metabolic improvements and direct antiinflammatory actions on the intestine.
-
GLP-1 medicines in arthritis
There is growing interest in the potential of GLP-1 medicines to modulate joint inflammation and pain in arthritis. In a sodium monoiodoacetate–induced model of osteoarthritis, a single intra-articular injection of 20 μg liraglutide reduced pain sensitivity and synovitis, in the absence of any changes in body weight (86). In addition, in IL-1β–stimulated mouse primary chondrocytes, treatment with 53.1 nM liraglutide downregulated Nos2, Cox2, and Tnf. In a mouse model of osteoarthritis induced by destabilization of the medial meniscus, glycoursodeoxycholic acid was found to mitigate disease progression, an effect associated with an increase in intestinal L cell numbers (87). Pharmacologically, intra-articular injection of 1 μM liraglutide twice weekly reduced cartilage degradation in mice. In vitro treatment with 10 or 100 nM liraglutide upregulated ACAN and downregulated ADAMTS5 protein levels in TNF-α–stimulated primary mouse chondrocytes. GLP-1R immunoreactivity has also been detected in mouse cartilage and synovium using immunofluorescence techniques. The expression and functional relevance of GLP-1R in joint tissues require further validation using knockout models. Nevertheless, these findings point to complex interaction between GLP-1 and the gut, brain, immune system, and gut microbiota in control of inflammation (Figure 3 and Figure 4), offering osteoarthritis as a relevant inflammation model to explore the systemic, integrative actions of GLP-1 medicines beyond metabolic control (Figure 4).
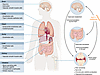 Figure 4
Figure 4Potential cellular targets mediating the antiinflammatory effects of GLP-1 medicines. GLP-1Rs are expressed at low levels in most peripheral organs and often in rare cell types. In osteoarthritis, a well-established inflammatory condition, GLP-1 medicines likely exert their effects through multiple pathways. These may include direct actions on joint tissues such as the synovium and cartilage. Interactions between GLP-1 medicines, the intestinal immune system including IELs, and the gut microbiota may also influence joint inflammation. Additionally, the central nervous system represents another site of GLP-1 action, contributing to the regulation of systemic inflammation through indirect mechanisms. Other common inflammatory diseases and metabolic complications may respond to GLP-1 medicines in a similar manner.
In a 68-week clinical trial involving individuals with obesity and knee osteoarthritis with moderate to severe pain (STEP-9), semaglutide treatment led to a greater reduction in the Western Ontario and McMaster Universities Osteoarthritis Index score (–41.7 points) as compared with placebo (–27.5 points) (88). While the contribution of weight loss remains unclear, this study, alongside emerging preclinical data from rodent models, suggests that GLP-1 medicines may offer efficacy in inflammation-driven diseases independent of the amelioration of metabolic dysfunction.
-
Antiinflammatory actions of GLP-1/GIP medicines
The GLP-1R/GIPR co-agonist tirzepatide is predicted to reduce inflammation, like other GLP-1R agonists. While GIPR agonism appears to enhance the efficacy of GLP-1R agonism for glucose control and weight loss, its impact on the antiinflammatory actions of GLP-1R agonism is less clear. For example, in Glp1rNes–/– mice, semaglutide loses its acute antiinflammatory effects against LPS-induced inflammation, yet tirzepatide still effectively prevents TNF-α induction, suggesting that tirzepatide does not rely exclusively on neuronal GLP-1R to exert this effect (22). In a mouse model of 5-fluorouracil–induced mucositis, daily treatment with 3 nmol/kg tirzepatide for 7 days, but not 41 μg/kg semaglutide, significantly reduced ileal neutrophil elastase staining (89). These findings suggest that tirzepatide possesses distinct antiinflammatory properties compared with semaglutide, likely due to its ability to act on the GIPR. Consistently, loss of GIPR signaling in mice is associated with enhanced adipose tissue inflammation, atherosclerosis, and mucositis (89–91). Tirzepatide also lowers CRP levels in clinical trials in people with heart failure with preserved ejection fraction, or OSA (19, 66). Future research will help clarify whether the affinity of tirzepatide for both GLP-1R and GIPR might contribute to distinct antiinflammatory effects in clinical settings.
-
Conclusions, limitations, and future directions
The success of GLP-1 medicines as a breakthrough in medical obesity treatment marks the culmination of over 4 decades of research into GLP-1 physiology and pharmacology. Recent clinical trials demonstrating the efficacy of newer GLP-1 medicines across a broader range of diseases raise a fundamental question in GLP-1 pharmacology: Is metabolic control alone sufficient to explain such broad efficacy? Evidence from clinical trials suggests that GLP-1 medicines exert clinical benefits even in individuals who do not experience substantial weight loss (25). This underscores the need to investigate non-metabolic mechanisms contributing to their efficacy, particularly their ability to modulate inflammation, a key driver of many chronic diseases in which these therapies show benefit.
Although GLP-1R expression is relatively low in many peripheral organs, GLP-1 medicines exert distinct antiinflammatory effects through both direct and indirect mechanisms that involve immune, vascular, and neural crosstalk (Figure 4). Distinguishing between metabolic and non-metabolic antiinflammatory effects of GLP-1 medicines will require a multifaceted approach, including complementary transgenic models, pair-feeding experiments to control for metabolic confounders, and interdisciplinary techniques that span physiology, neuroscience, and immunology.
While GLP-1 medicines demonstrate multiple antiinflammatory effects, their use has also been associated with certain inflammation-related adverse events, notably cholecystitis. Concerns regarding pancreatitis have not been substantiated in large cardiovascular outcome trials (92, 93). Given their antiinflammatory properties, there has been some speculation that GLP-1 medicines could impair host immune defenses, but current clinical evidence does not indicate an increased risk of infections or obesity-related cancer (94). Whether and how GLP-1 medicines might impact the risk of cancer or infections when used in combination with other immunomodulatory treatments is not known.
As new GLP-1 medicines advance through clinical trials, it will be crucial to expand our understanding of their antiinflammatory mechanisms in order to optimize therapeutic use. How does adding glucagon, GIP, or amylin modify the antiinflammatory actions of GLP-1 medicines? What are the key immune cell types and pathways that link GLP-1R signaling to the reduction of inflammation? Answering these questions will guide the development of pharmacotherapies with targeted antiinflammatory effects, perhaps enabling precision medicine approaches by aligning specific drugs with appropriate patient populations, and broadening the clinical applications of GLP-1 medicines, some of which may not yet be fully recognized.
-
Acknowledgments
DJD is supported, in part, by a Banting and Best Diabetes Centre–Novo Nordisk Chair in Incretin Biology, a Sinai Health–Novo Nordisk Foundation Fund in Regulatory Peptides, Canadian Institutes of Health Research grants 154321 and 19204, and Diabetes Canada–Canadian Cancer Society grant OG-3-24-5819-DD.
Address correspondence to: Daniel J. Drucker, Lunenfeld-Tanenbaum Research Institute, Mt. Sinai Hospital, 600 University Avenue Mailbox 39 TCP 5-1004, Toronto, Ontario M5G 1X5, Canada. Phone: 416.361.2661; Email: drucker@lunenfeld.ca.
-
Footnotes
Conflict of interest: DJD has received consulting fees from Anylam, Amgen, AstraZeneca Inc., Crinetics, Eli Lilly, Insulet, Kallyope, Metsara, and Pfizer Inc. and speaking fees from Novo Nordisk. Mount Sinai Hospital has received investigator-initiated grant support from Amgen, Eli Lilly, Novo Nordisk, Pfizer Inc., and Zealand Pharmaceuticals Inc. to support preclinical studies in the Drucker laboratory.
Copyright: © 2025, Wong et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(21):e194751.https://doi.org/10.1172/JCI194751.
-
References
- Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216.
- Frias JP, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402(10403):720–730.
- Jastreboff AM, et al. Triple-hormone-receptor agonist retatrutide for obesity — a phase 2 trial. N Engl J Med. 2023;389(6):514–526.
- Sanyal AJ, et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30(7):2037–2048.
- Kahles F, et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. 2014;63(10):3221–3229.
- Lebrun LJ, et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 2017;21(5):1160–1168.
- Panaro BL, et al. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol Metab. 2020;37:100990.
- Bang-Berthelsen CH, et al. GLP-1 induces barrier protective expression in Brunner’s glands and regulates colonic inflammation. Inflamm Bowel Dis. 2016;22(9):2078–2097.
- Brodersen K, et al. Prolonged lipopolysaccharide-induced illness elevates glucagon-like peptide-1 and suppresses peptide YY: a human-randomized cross-over trial. Physiol Rep. 2022;10(18):e15462.
- Brakenridge SC, et al. Persistently elevated glucagon-like peptide-1 levels among critically ill surgical patients after sepsis and development of chronic critical illness and dismal long-term outcomes. J Am Coll Surg. 2019;229(1):58–67.
- Lebherz C, et al. GLP-1 levels predict mortality in patients with critical illness as well as end-stage renal disease. Am J Med. 2017;130(7):833–841.
- Hansell CE, et al. Glucagon-like peptide-1 is prognostic of mortality in acute respiratory failure. Crit Care Explor. 2025;7(4):e1247.
- Lang Lehrskov L, et al. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018;27(6):1201–1211.
- Jin H, et al. A body-brain circuit that regulates body inflammatory responses. Nature. 2024;630(8017):695–703.
- Athauda D, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–1675.
- Pi-Sunyer X, et al. A randomized, controlled trial of 3.0 Mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
- Mosenzon O, et al. Impact of semaglutide on high-sensitivity C-reactive protein: exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol. 2022;21(1):172.
- Verma S, et al. Effects of once-weekly semaglutide 2.4 mg on C-reactive protein in adults with overweight or obesity (STEP 1, 2, and 3): exploratory analyses of three randomised, double-blind, placebo-controlled, phase 3 trials. EClinicalMedicine. 2023;55:101737.
- Malhotra A, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. 2024;391(13):1193–1205.
- Selvin E, et al. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167(1):31–39.
- Rakipovski G, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE–/– and LDLr–/– mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3(6):844–857.
- Wong CK, et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 2024;36(1):130–143.
- Chaudhuri A, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97(1):198–207.
- Rodbard HW, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–2281.
- Maretty L, et al. Proteomic changes upon treatment with semaglutide in individuals with obesity. Nat Med. 2025;31(1):267–277.
- McLean BA, et al. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr Rev. 2021;42(2):101–132.
- Lee YS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55(9):2456–2468.
- Somm E, et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res. 2021;227:75–88.
- McLean B, et al. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic vs. hepatic actions of semaglutide. JCI Insight. 2021;6(22):e153732.
- Trevaskis JL, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):762–772.
- Moschovaki Filippidou F, et al. Glucagon-like peptide-1 receptor agonism improves nephrotoxic serum nephritis by inhibiting T-cell proliferation. Am J Pathol. 2020;190(2):400–411.
- Noyan-Ashraf MH, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127(1):74–85.
- Yusta B, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537–2549.
- Wong CK, et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 2022;34(10):1514–1531.
- Hadjiyanni I, et al. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53(4):730–740.
- Ben Nasr M, et al. Glucagon-like peptide 1 receptor is a T cell-negative costimulatory molecule. Cell Metab. 2024;36(6):1302–1319.
- Panjwani N, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2013;154(1):127–139.
- Helmstadter J, et al. Endothelial GLP-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler Thromb Vasc Biol. 2020;40(1):145–158.
- Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor—or not? Endocrinology. 2013;154(1):4–8.
- Baggio LL, et al. GLP-1 receptor expression within the human heart. Endocrinology. 2018;159(4):1570–1584.
- Wong C, et al. Reassessment of antibody-based detection of the murine T cell GLP-1 receptor. Cell Metab. 2025;37(9):1783–1788.
- Yusta B, et al. GLP-1R signaling does not modify the severity of experimental graft versus host disease. [published online August 20, 2025]. Mol Metab. https://doi.org/10.1016/j.molmet.2025.102235. View this article via: PubMed Google Scholar
- Hogan AE, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54(11):2745–2754.
- Akhtar A, et al. Bioengineering the metabolic network of CAR T cells with GLP-1 and Urolithin A increases persistence and long-term anti-tumor activity. Cell Rep Med. 2025;6(3):102021.
- Wilson DM, et al. Hallmarks of neurodegenerative diseases. Cell. 2023;186(4):693–714.
- Yun SP, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24(7):931–938.
- Zhou L, et al. Myeloid cell modulation by a GLP-1 receptor agonist regulates retinal angiogenesis in ischemic retinopathy. JCI Insight. 2021;6(23):e93382.
- Timper K, et al. GLP-1 receptor signaling in astrocytes regulates fatty acid oxidation, mitochondrial integrity, and function. Cell Metab. 2020;31(6):1189–1205.
- Heiss CN, et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021;35(8):109163.
- McClean PL, et al. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer’s disease. Eur J Pharmacol. 2010;630(1-3):158–162.
- Forny Germano L, et al. The GLP-1 medicines semaglutide and tirzepatide do not alter disease-related pathology, behaviour or cognitive function in 5XFAD and APP/PS1 mice. Mol Metab. 2024;89:102019.
- Gharagozloo M, et al. The effects of NLY01, a novel glucagon-like peptide-1 receptor agonist, on cuprizone-induced demyelination and remyelination: challenges and future perspectives. Neurotherapeutics. 2023;20(4):1229–1240.
- Meissner WG, et al. Trial of lixisenatide in early Parkinson’s disease. N Engl J Med. 2024;390(13):1176–1185.
- McGarry A, et al. Safety, tolerability, and efficacy of NLY01 in early untreated Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2024;23(1):37–45.
- Vijiaratnam N, et al. Exenatide once a week versus placebo as a potential disease-modifying treatment for people with Parkinson’s disease in the UK: a phase 3, multicentre, double-blind, parallel-group, randomised, placebo-controlled trial. Lancet. 2025;405(10479):627–636.
- Payne S, et al. Endothelial-specific Cre mouse models. Arterioscler Thromb Vasc Biol. 2018;38(11):2550–2561.
- McLean BA, et al. Glucagon-like Peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol Metab. 2022;66:101641.
- Lu HI, et al. Exendin-4 therapy still offered an additional benefit on reducing transverse aortic constriction-induced cardiac hypertrophy-caused myocardial damage in DPP-4 deficient rats. Am J Transl Res. 2016;8(2):778–798.View this article via: PubMed Google Scholar
- He W, et al. Semaglutide ameliorates pressure overload-induced cardiac hypertrophy by improving cardiac mitophagy to suppress the activation of NLRP3 inflammasome. Sci Rep. 2024;14(1):11824.
- Marso SP, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844.
- Gerstein HC, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130.
- Lincoff AM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–2232.
- Nidorf SM, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–1847.
- Ridker PM, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131.
- Kosiborod MN, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–1084.
- Packer M, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. 2025;392(5):427–437.
- Wang Y, et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br J Pharmacol. 2014;171(3):723–734.
- Sanyal AJ, et al. Phase 3 trial of semaglutide in metabolic dysfunction-associated steatohepatitis. N Engl J Med. 2025;392(21):2089–2099.
- Loomba R, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(6):511–522.
- Sourris KC, et al. Glucagon-like peptide-1 receptor signaling modifies the extent of diabetic kidney disease through dampening the receptor for advanced glycation end products-induced inflammation. Kidney Int. 2024;105(1):132–149.
- Jensen EP, et al. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol. 2015;308(8):F867–F877.
- Bjornholm KD, et al. Decreased expression of the GLP-1 receptor after segmental artery injury in mice. J Endocrinol. 2021;248(3):289–301.
- Tuttle KR, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617.
- Tuttle KR, et al. Indicators of kidney fibrosis in patients with type 2 diabetes and chronic kidney disease treated with dulaglutide. Am J Nephrol. 2023;54(1-2):74–82.
- Perkovic V, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391(2):109–121.
- Zhou W, et al. Glucagon-like peptide-1 receptor mediates the beneficial effect of liraglutide in an acute lung injury mouse model involving the thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2020;319(3):568–578.
- Sato T, et al. GLP-1 receptor signaling differentially modifies the outcomes of sterile vs viral pulmonary inflammation in male mice. Endocrinology. 2020;161(12):bqaa201.
- Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358(3):219–224.
- Scirica BM, et al. The effect of semaglutide on mortality and COVID-19-related deaths: an analysis from the SELECT Trial. J Am Coll Cardiol. 2024;84(17):1632–1642.
- Blackman A, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40(8):1310–1319.
- Song Y, et al. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 2019;30(5):976–986.
- Varin EM, et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 2019;27(11):3371–3384.
- Villumsen M, et al. GLP-1 based therapies and disease course of inflammatory bowel disease. EClinicalMedicine. 2021;37:100979.
- Gorelik Y, et al. GLP-1 analog use is associated with improved disease course in inflammatory bowel disease: a report from the Epi-IIRN. J Crohns Colitis. 2025;19(4):jjae160.
- Singh S, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110–121.
- Meurot C, et al. Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts analgesic, anti-inflammatory and anti-degradative actions in osteoarthritis. Sci Rep. 2022;12(1):1567.
- Yang Y, et al. Osteoarthritis treatment via the GLP-1-mediated gut-joint axis targets intestinal FXR signaling. Science. 2025;388(6742):eadt0548.
- Bliddal H, et al. Once-weekly semaglutide in persons with obesity and knee osteoarthritis. N Engl J Med. 2024;391(17):1573–1583.
- Hammoud R, et al. Glucose-dependent insulinotropic polypeptide receptor signaling alleviates gut inflammation in mice. JCI Insight. 2024;10(3):e174825.
- Mantelmacher FD, et al. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat Metab. 2019;1(1):58–69.
- Pujadas G, et al. Genetic disruption of the Gipr in Apoe–/– mice promotes atherosclerosis. Mol Metab. 2022;65:101586.
- Drucker DJ. Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care. 2024;47(11):1873–1888.
- Chiang CH, et al. Glucagon-like peptide-1 receptor agonists and gastrointestinal adverse events: a systematic review and meta-analysis [published online June 9, 2025]. Gastroenterology. https://doi.org/10.1053/j.gastro.2025.06.003.
- Wang L, et al. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw Open. 2024;7(7):e2421305.
-
Version history
- Version 1 (November 3, 2025): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Review Series
Clinical innovation and scientific progress in GLP-1 medicine
-
The science of safety: adverse effects of GLP-1 receptor agonists as glucose-lowering and obesity medications
Ryan J. Jalleh et al. -
GLP-1RA precision medicine in people with type 2 diabetes: current insights and future prospects
Pedro Cardoso et al. -
GLP-1 receptor agonists and cancer: current clinical evidence and translational opportunities for preclinical research
Estefania Valencia-Rincón et al. -
GLP-1 physiology and pharmacology along the gut-brain axis
Lisa R. Beutler -
The promise of GLP-1 receptor agonists for neurodegenerative diseases
Dilan Athauda et al. -
GLP-1 and the cardiovascular system
Florian Kahles et al. -
GLP-1 agonists in the treatment of chronic kidney disease in type 2 diabetes and obesity
Mark E. Cooper et al. -
Antiinflammatory actions of glucagon-like peptide-1–based therapies beyond metabolic benefits
Chi Kin Wong et al. -
Signaling architecture of the glucagon-like peptide-1 receptor
Gregory Austin et al.
Metrics
Go to
- Top
- Abstract
- GLP-1–producing L cells as pathogen and inflammation sensors
- Weight loss–independent antiinflammatory effects of GLP-1 medicines
- GLP-1R expression in the immune system
- Indirect actions of GLP-1 medicines on immune function
- GLP-1 medicines in neuroinflammation
- GLP-1 medicines in cardiovascular inflammation
- GLP-1 medicines in liver inflammation
- GLP-1 medicines in kidney inflammation
- GLP-1 medicines in pulmonary inflammation
- GLP-1 medicines in intestinal inflammation
- GLP-1 medicines in arthritis
- Antiinflammatory actions of GLP-1/GIP medicines
- Conclusions, limitations, and future directions
- Acknowledgments
- Footnotes
- References
- Version history



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)



