Advertisement
Review Series
Open Access |  10.1172/JCI194745
10.1172/JCI194745
The promise of GLP-1 receptor agonists for neurodegenerative diseases
Dilan Athauda,1,2 Nigel H. Greig,3 Wassilios G. Meissner,4,5,6 Thomas Foltynie,2 and Sonia Gandhi1,2
1The Francis Crick Institute, London, United Kingdom.
2Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, United Kingdom.
3Drug Design and Development Section, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
4CHU Bordeaux, Service de Neurologie des Maladies Neurodégénératives, IMNc, Bordeaux, France.
5University de Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
6Department of Medicine, University of Otago, Christchurch, and New Zealand Brain Research Institute, Christchurch, New Zealand.
Address correspondence to: Dilan Athauda, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, United Kingdom. Email: dilan.athauda@crick.ac.uk.
Find articles by Athauda, D. in: PubMed | Google Scholar
1The Francis Crick Institute, London, United Kingdom.
2Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, United Kingdom.
3Drug Design and Development Section, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
4CHU Bordeaux, Service de Neurologie des Maladies Neurodégénératives, IMNc, Bordeaux, France.
5University de Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
6Department of Medicine, University of Otago, Christchurch, and New Zealand Brain Research Institute, Christchurch, New Zealand.
Address correspondence to: Dilan Athauda, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, United Kingdom. Email: dilan.athauda@crick.ac.uk.
Find articles by Greig, N. in: PubMed | Google Scholar
1The Francis Crick Institute, London, United Kingdom.
2Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, United Kingdom.
3Drug Design and Development Section, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
4CHU Bordeaux, Service de Neurologie des Maladies Neurodégénératives, IMNc, Bordeaux, France.
5University de Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
6Department of Medicine, University of Otago, Christchurch, and New Zealand Brain Research Institute, Christchurch, New Zealand.
Address correspondence to: Dilan Athauda, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, United Kingdom. Email: dilan.athauda@crick.ac.uk.
Find articles by
Meissner, W.
in:
PubMed
|
Google Scholar
|

1The Francis Crick Institute, London, United Kingdom.
2Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, United Kingdom.
3Drug Design and Development Section, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
4CHU Bordeaux, Service de Neurologie des Maladies Neurodégénératives, IMNc, Bordeaux, France.
5University de Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
6Department of Medicine, University of Otago, Christchurch, and New Zealand Brain Research Institute, Christchurch, New Zealand.
Address correspondence to: Dilan Athauda, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, United Kingdom. Email: dilan.athauda@crick.ac.uk.
Find articles by Foltynie, T. in: PubMed | Google Scholar
1The Francis Crick Institute, London, United Kingdom.
2Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, United Kingdom.
3Drug Design and Development Section, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, NIH, Baltimore, Maryland, USA.
4CHU Bordeaux, Service de Neurologie des Maladies Neurodégénératives, IMNc, Bordeaux, France.
5University de Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
6Department of Medicine, University of Otago, Christchurch, and New Zealand Brain Research Institute, Christchurch, New Zealand.
Address correspondence to: Dilan Athauda, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, United Kingdom. Email: dilan.athauda@crick.ac.uk.
Find articles by Gandhi, S. in: PubMed | Google Scholar
Published February 16, 2026 - More info
J Clin Invest. 2026;136(4):e194745. https://doi.org/10.1172/JCI194745.
© 2026 Athauda et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs), established therapies for type 2 diabetes and obesity, are increasingly recognized for their potential in neurodegenerative diseases. Preclinical studies across diverse neurodegenerative conditions consistently demonstrate neuroprotective effects of GLP-1RAs, including reduced protein aggregation, enhanced autophagy, improved mitochondrial function, suppression of neuroinflammation, and preservation of synaptic integrity. Epidemiological analyses further suggest reduced incidence of dementia, Parkinson disease, and multiple sclerosis among long-term GLP-1RA users. Early human trials provide signals of target engagement, such as preserved cerebral glucose metabolism, altered inflammatory biomarkers, and slowed brain atrophy, although clinical outcomes to date remain mixed and trials in rarer disorders are sparse. Translation is constrained by uncertainty around optimal molecule choice, CNS penetrance, tolerability, adherence, and heterogeneity of response. Furthermore, next-generation dual and triple agonists may offer enhanced efficacy but remain untested in neurodegeneration. Conceptually, GLP-1RAs share pleiotropic effects with exercise — one of the few interventions with proven disease-modifying potential — by enhancing insulin signaling, stabilizing mitochondria, reducing inflammation, and promoting synaptic plasticity. This overlap highlights their promise as “pharmacological analogues of exercise,” and underscores the need for biomarker-driven, disease-specific trials to establish whether GLP-1RAs can deliver durable disease modification across the spectrum of neurodegenerative diseases.
-
Introduction
Neurodegenerative diseases (NDDs) such as Alzheimer disease (AD), Parkinson disease (PD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), Huntington disease (HD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) are collectively characterized by progressive neuronal loss leading to impairments in cognition, motor control, and behavior. Their prevalence is rising sharply and is projected to surpass cancer as the second leading cause of death by 2040 (1). Although each condition has distinct pathological features, they converge on shared, interlinked biological “hallmarks”: pathological protein aggregation, synaptic and network dysfunction, impaired proteostasis, cytoskeletal abnormalities, mitochondrial dysfunction, inflammation, and ultimately neuronal death.
A substantial body of evidence now demonstrates that these hallmarks are strongly modulated by systemic metabolic health. Type 2 diabetes mellitus (T2DM), defined by insulin resistance (IR), hyperglycemia, and altered lipid metabolism, increases the risk of several NDDs (2). Epidemiological studies show that people with T2DM have approximately double the risk of AD (3); an approximately 40% increased risk of developing PD (4–6); and are approximately 1.5 times more likely to develop MS (7), with longer duration and poorer glycemic control conferring greater risk. Conversely, T2DM appears to reduce ALS risk in older adults (8–10), though this may reflect effects of insulinotropic medications rather than diabetes itself (11). Importantly, comorbid T2DM or elevated peripheral IR accelerate disease progression in AD and PD independently of vascular disease, with higher peripheral IR predicting conversion from mild cognitive impairment to AD (12, 13) and faster motor and cognitive decline in PD (14, 15), thereby implicating IR as a modifier of NDD biology rather than a simple comorbidity.
A convergent mechanism linking these conditions is brain insulin resistance (BIR). In the CNS, insulin regulates synaptic plasticity, mitochondrial function, neuronal survival, and glial activation. In BIR, inhibitory serine phosphorylation of IRS-1 (S312/S616/S636) disrupts PI3K/Akt signaling, deactivating downstream insulin signaling. This leads to impaired synaptic plasticity and long-term potentiation (LTP) (16, 17), tau hyperphosphorylation (18–21), reduced clearance of Aβ (22–24) and α-synuclein (25, 26), and microglial activation (27, 28), and impaired mitochondrial function (29, 30). These processes form a self-reinforcing loop; IR-driven mitochondrial stress and impaired mitophagy exacerbate Aβ (31, 32), tau (18), and α-synuclein pathology (25), which in turn further impairs insulin signaling (33). BIR has been demonstrated in postmortem studies of patients with PD (34), AD (35), DLB (36), and MSA (37), associated with dopaminergic depletion in the caudate and ventral striatum (14, 38), frontotemporal cortical atrophy (39, 40), and cerebral glucose hypometabolism (41). Similar metabolic defects occur in HD (42, 43) and MS (44).
These insights have intensified interest in therapies that restore central metabolic resilience. Among these, glucagon-like peptide-1 receptor agonists (GLP-1RAs) — developed to treat T2DM and obesity — are uniquely positioned as potential disease-modifying agents. GLP-1 is secreted by intestinal L cells and by preproglucagon neurons in the nucleus tractus solitarius, which project widely to areas governing cognition, motor control, reward, autonomic regulation, and energy homeostasis (45, 46). GLP-1Rs are expressed in the hypothalamus, hippocampus, cortex, and brainstem, as well as limbic and reward-related circuits (47). Activation of GLP-1R engages cAMP/PKA and PI3K/Akt pathways overlapping with insulin receptor signaling. Through these mechanisms, GLP-1RAs activate multiple pathways, reduce inflammation, improve mitochondrial function, modulate proteostasis, and promote synaptic plasticity (48–52), a profile well suited to the multifactorial nature of NDDs.
Across diverse preclinical models and early human trials, GLP-1RAs demonstrate broad neuroprotective effects and signals of clinical benefit, with next-generation agents aiming to further enhance central penetrance and potency. Nevertheless, important uncertainties remain, including the degree of brain penetrance across individual agents, the relative contributions of central versus peripheral mechanisms, biomarkers of target engagement, and the patient subgroups most likely to benefit.
In this Review, we evaluate the translational evidence supporting GLP-1RAs as candidate disease-modifying therapies for NDDs, describe mechanistic pathways linking metabolic dysfunction to neuronal vulnerability, and outline remaining challenges and research priorities in this rapidly evolving field.
-
Central mechanisms of GLP-1RAs in neurodegeneration
Regulation of protein aggregation and proteostasis. Pathological protein aggregation is a unifying hallmark of NDDs: Aβ and tau in AD, α-synuclein in PD and MSA, mutant huntingtin in HD, and TDP-43 in ALS. These assemblies impair multiple cellular processes including synaptic, mitochondrial, and autophagy–lysosomal function, and activate inflammation, making restoration of proteostasis a central therapeutic goal.
GLP-1R activation engages PI3K/Akt and cAMP/PKA pathways that converge on kinases that modulate autophagy, including mTOR, AMPK, and transcription factor EB (TFEB). GLP-1RAs also upregulate sirtuin-1 (SIRT1) indirectly (53), which deacetylates autophagy-related proteins and promotes autophagosome formation. Complementary improvements in mitochondrial function and redox homeostasis further support proteostasis. Through these mechanisms, GLP-1RAs promote protein clearance and reduce cellular stress that drives aggregation (Figure 1).
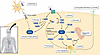 Figure 1
Figure 1Central mechanisms of GLP-1RAs. GLP-1RAs engage PI3K/AKT and cAMP/PKA pathways to modulate autophagy and improve mitochondrial function and redox homeostasis. Collectively, these effects promote protein clearance and reduce cellular stress that drives aggregation. GLP-1RAs also exert direct antiinflammatory effects on glia. These pleiotropic actions converge to preserve neuronal viability and network function.
Preclinical evidence shows broad anti-aggregation effects of GLP-1RAs. GLP-1RAs reduce tau phosphorylation and Aβ plaque burden in AD models (54–56), attenuate α-synuclein pathology in PD models (57) and in induced pluripotent stem cell–derived (iPSC-derived) dopaminergic neurons with PD-linked SNCA mutations (26), and lower mutant huntingtin aggregates in a mouse model of HD (58). Data in ALS models are less robust but suggest GLP-1RAs induce partial mitigation of TDP-43 pathology (59).
Clinical evidence supporting these effects remains limited. In a phase II trial of 60 individuals with PD, 48 weeks of exenatide, a first-generation GLP-1RA, was associated with reduced oligomeric α-synuclein in the cerebrospinal fluid (CSF) compared with placebo, alongside motor benefits (26, 60). However, these results were not replicated in a larger multicenter trial conducted over 96 weeks (61). In a study of 18 people with AD, exenatide treatment for 18 months was associated with lower plasma-sourced, neuron-derived extracellular vesicle (NDEV) Aβ42 levels, although all other fluid biomarkers remained unchanged (62). Consistent effects on tau- or p-tau–related biomarkers remain unproven.
Overall, these results indicate GLP-1RAs can influence a number of proteinopathies across disease models, although more understanding is required to determine when in the course of disease proteostasis can be modulated to bring about reduced neuronal loss and clinical benefit. Translation to clinical benefit will require biomarker-driven studies incorporating fluid and imaging measures of proteostasis.
Restoring energy metabolism and mitochondrial function. Defective energy metabolism is a consistent feature of NDDs, encompassing mitochondrial dysfunction, altered substrate utilization, and impaired hypothalamic regulation. Familial PD genes (Parkin [PRKN, also known as PARK2] and PINK1) implicate impaired mitophagy in disease progression (63), fluorodeoxyglucose PET (FDG-PET) studies show consistent cerebral glucose hypometabolism (64), and hypothalamic dysfunction has been linked to ALS and HD (65). These abnormalities support the concept that energy homeostasis failure is an early and upstream driver of neurodegeneration.
GLP-1RAs can target these abnormalities at multiple levels. Via AMPK/SIRT1/PGC-1α signaling, they promote mitochondrial biogenesis (66–68) and enhance PINK1- and Parkin-mediated mitophagy. These effects, together with upregulation of antioxidant modulators such as Nrf2, reduce ROS generation (69–71). In glia, AMPK/SIRT1/PGC-1α signaling counters proinflammatory metabolic shifts, e.g., glycolytic reliance in astrocytes, improving neuronal support and functional outcomes in transgenic AD mice (72). Furthermore, GLP-1RAs can affect hypothalamic regulation; for example, liraglutide and exendin-4 normalized glucose metabolism and prevented weight loss in a transgenic HD model (58).
Clinical data are limited but support these effects. In patients with AD, 6 months of liraglutide increased blood-brain glucose transfer (likely via GLUT upregulation) (73), and stabilized cerebral glucose metabolism on FDG-PET (74). Whether these metabolic effects translate into durable clinical benefit or arise from direct CNS actions versus systemic metabolic improvement remains uncertain.
Mitigating DNA damage and RNA dysregulation. Neurons are highly vulnerable to DNA damage caused by ROS from mitochondrial metabolism, while upstream defects in RNA processing involving defects in splicing, transport, and stress granule dynamics can disrupt protein homeostasis and promote aggregation. These nucleic acid defects are central features of multiple NDDs.
GLP-1RAs have recently been shown to mitigate these processes by enhancing DNA repair and stabilizing RNA metabolism. GLP-1RAs can enhance neuronal DNA repair capacity through the base excision repair pathway (75). In an ischemic stroke model, exendin-4 enhanced the activation of PI3K/Akt/CREB signaling, leading to upregulation of APE1, a key enzyme responsible for resolving oxidized bases and single-strand breaks, which reduced nuclear DNA fragmentation and preserved neuronal viability (75, 76). In addition, by improving mitochondrial function through AMPK/SIRT1 signaling, GLP-1RAs reduce ROS generation, indirectly lowering DNA and RNA damage burden.
Overall, GLP-1RAs clearly enhance DNA repair and reduce oxidative stress, but their impact on RNA-processing defects remains uncertain. Establishing their impact on nucleic acid pathology will require studies in proteinopathies such as ALS, FTD, and HD.
Regulating glial activation and neuroinflammation. Neuroinflammation is a key hallmark of NDDs, characterized by chronic activation of microglia and astrocytes, excessive cytokine release, and infiltration of peripheral immune cells. Although inflammatory responses are initially protective, sustained glial activation promotes oxidative stress and synaptic injury, and accelerates protein aggregation, which itself exacerbates inflammation, perpetuating a vicious cycle.
GLP-1Rs are expressed on glial cells (77, 78), which allows GLP-1RAs to exert direct antiinflammatory effects. Activation of GLP-1R enhances Akt-dominant (neuroprotective) signaling and avoids prolonged MAPK/JNK signaling that drives cytokine release. GLP-1R–mediated activation of the cAMP/PKA and PI3K/Akt pathways inhibits NF-κB, and thus reduces expression of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 (79). GLP-RAs also activate AMPK, and through downstream SIRT1 also inhibit NF-κB translocation and thus indirectly suppress proinflammatory gene transcription (80), leading to promotion of autophagy and mitochondrial stability, thereby reducing damage-associated molecular patterns that sustain microglial activation (81). In addition, GLP-1RAs inhibit the NLRP3 inflammasome (which is implicated in multiple NDDs; ref. 82), diminishing activation of its downstream cytokine cascade and reducing pyroptosis (83). Another emerging mechanism involves the cGAS/STING pathway; mitochondrial stress and defective autophagy cause cytosolic leakage of mtDNA, activating STING and proinflammatory cytokines, and STING dysfunction has been identified as a key player in NDDs (84, 85). By enhancing autophagy and stabilizing mitochondria indirectly via AMPK and SIRT, GLP-1RAs may also act as indirect suppressors of pathological STING activation.
Preclinical models support these mechanisms. GLP-1RAs reduce microglial activation and cytokine release in PD (86), AD, and MS models (87), with parallel improvements in behavior. Clinical evidence, although still emerging, aligns with these findings. In people with T2DM, exenatide treatment reduced serum levels of inflammatory proteins linked to AD risk (88), and studies with liraglutide or sitagliptin (a DPP-IV inhibitor that elevates GLP-1 levels) reported lower cytokine levels and modest cognitive improvements (89). However, direct biomarker evidence in NDD populations is limited, and the relative contributions of central versus systemic immune modulation remain uncertain.
Collectively, GLP-1RAs exert immunomodulatory effects through multiple mechanisms, including reducing NF-κB and inflammasome signaling, activating SIRT1/AMPK networks, and attenuating inflammatory glial phenotypes. Key uncertainties include how best to measure these effects in vivo and the disease stages at which inflammatory modulation is most beneficial.
Restoring synaptic function and network integrity. Synaptic dysfunction is one of the earliest features of NDDs, preceding neuronal loss and driving network dysfunction. Normal synaptic activity can be disrupted by mitochondrial dysfunction, aberrant glutamate signaling, and toxic protein aggregates, resulting in synaptic hyperexcitability, loss of dendritic spines, and impaired plasticity.
GLP-1RAs act on several of these mechanisms. Activation of cAMP/PKA/CREB signaling upregulates brain-derived neurotrophic factor (BDNF) and stabilizes dendritic spines (90). In hippocampal preparations, exendin-4 and liraglutide restore LTP suppressed by Aβ and tau (56, 91), and GLP-1RAs also normalize calcium handling and limit glutamate excitotoxicity (79, 92, 93). By enhancing autophagy and proteostasis, they facilitate clearance of toxic protein oligomers at pre- and postsynaptic sites, thereby preserving function (94, 95). Together, these effects are associated with preservation of synaptic proteins such as synaptophysin and PSD-95 in AD and PD models (96).
In human studies, liraglutide treatment in AD enhanced cerebral glucose metabolism and showed signals of preserved network activity (97). However, direct evidence for true synaptic rescue remains limited.
Overall, GLP-1RAs may affect multiple pathways that influence synaptic integrity, and these effects, though not yet fully validated in humans, provide a basis for their potential to slow network dysfunction across NDDs.
Restoring gut-immune-brain homeostasis. The gut microbiota–immune-brain axis is a complex bidirectional network connecting microbial communities, the vagus nerve, the neuroendocrine system, and immune pathways (Figure 2). Dysbiosis, characterized by a reduction in microbiota diversity, leads to loss of “beneficial” bacteria that produce antiinflammatory short-chain fatty acids (SCFAs) and has been observed across AD, PD, and MS (98–101). This imbalance promotes increased intestinal and blood-brain barrier (BBB) permeability, allowing inflammatory molecules from the intestine to reach the brain and vice versa, and can cause systemic and neuroinflammation; and facilitating entry of microbial metabolites into the CNS. For example, bacterial amyloids, such as E. coli curli proteins, can directly interact with human amyloids, and potentially accelerate aggregation of human Aβ and α-synuclein in AD and PD (102, 103). In addition, the gut-derived metabolite imidazole propionate has been shown to drive α-synuclein pathology in preclinical and patient-derived PD models, providing a direct link between dysbiosis and α-synuclein aggregation (103). Dysbiosis in PD toxin models is also accompanied by reduced GLP-1 levels in colon and brain, reversible with probiotics, further linking this axis to disease vulnerability (104).
 Figure 2
Figure 2Modulation of the gut-immune-brain axis by GLP-1RAs. GLP-1Rs are expressed throughout the gut-immune-brain network, enabling GLP-1RAs to restore homeostasis at multiple levels. GLP-1RAs reshape microbiota composition toward beneficial taxa. GLP-1RAs also improve gut and BBB integrity, preventing leakage of microbial metabolites into the CNS and reducing inflammation. Improved barrier function may also prevent bacterial amyloids, such as E. coli curli proteins, from interacting with human amyloids and accelerating their aggregation. Together, these actions support homeostasis across the gut-brain axis. IEL, intraepithelial lymphocyte.
GLP-1Rs are expressed throughout the gut-immune-brain network, enabling GLP-1RAs to restore homeostasis at multiple levels. At the gut barrier, GLP-1RAs improve epithelial integrity, reduce tight junction permeability, and suppress endotoxin translocation, thereby lowering systemic LPS-driven inflammation (105). GLP-1RAs also shift microbiota composition, increasing beneficial SCFA-producing taxa such as Akkermansia and Bifidobacterium, which may alleviate systemic inflammation (106–108). Within the immune system, they inhibit NF-κB and NLRP3 inflammasome activation, promote antiinflammatory IL-10 release, and promote macrophages and microglia toward antiinflammatory phenotypes (109, 110) (Figure 3), which could indirectly reduce cross-seeding of Aβ and α-synuclein initiated in the gut.
 Figure 3
Figure 3Systemic antiinflammatory effects of GLP-1RAs. GLP-1RAs can act directly on GLP-1Rs expressed on immune cells. In macrophages, this inhibits polarization toward an M1-like state, and promotes production of IL-10. In T cells, GLP-1R activation decreases differentiation into Th1 and Th17 cells, and promotes differentiation toward Th2 and Treg identities.
Together, these pleiotropic effects highlight the gut-immune-brain axis as both a site of vulnerability and a therapeutic target in NDDs. However, clinical data remain scarce, and it is unclear whether microbiome changes reflect direct GLP-1R signaling or secondary metabolic effects. Defining these relationships will be essential for translational progress.
-
GLP-1 therapies in neurodegenerative diseases
As described above, GLP-1RAs engage multiple convergent pathways implicated across NDDs, from proteostasis and mitochondrial quality control to neuroinflammation, synapse function, and gut-brain-immune interactions. The next step is to consider how these pleiotropic actions translate within specific disease contexts (Table 1).
AD. GLP-1RAs consistently demonstrate neuroprotective effects in AD models. Exenatide, lixisenatide, liraglutide, dulaglutide, and semaglutide have been shown to improve learning and memory while reducing Aβ/tau pathology, neuroinflammation, and synaptic/metabolic deficits in rodent models of sporadic toxin-induced neuronal injury (Aβ, streptozotocin, okadaic acid) (54, 111–120), and in multiple transgenic strains (5×FAD, 3×Tg, APP/PS1, Tg2576, APP/PS1/Tau) (121, 121–133). Human AD brain organoids treated with semaglutide similarly showed reduced Aβ, phosphorylated tau, and GFAP expression (134). These effects are attributed to restoration of neuronal insulin signaling, inhibition of GSK3β, reducing NF-κB/NLRP3-induced inflammation, and normalization of mitochondrial function and mitophagy. Notably, some studies report a lack of response (135).
Emerging human data suggest that GLP-1R modulation is relevant to AD risk and pathology. GLP1R polymorphisms (rs10305420, rs6923761) associate with increased AD risk and lower levels of CSF Aβ42/40, and higher levels of p-tau (136). A meta-analysis of 23 clinical studies showed GLP-1RA use was associated with reduced dementia incidence (137), and large retrospective cohorts report lower AD risk in obese patients treated with GLP-1RAs (138). In addition, indirect evidence from a peripheral proteomic study of 2,000 obese individuals prescribed semaglutide showed that semaglutide downregulated tenascin-C (TNC) and upregulated progranulin (GRN), both of which may reduce AD-related pathology (139).
A number of small clinical trials directly evaluating GLP-1RAs in AD have been completed. Early small trials of liraglutide (n = 38) and exenatide (n = 18) reported no significant cognitive or biomarker effects, although exploratory analyses indicated preserved cerebral glucose metabolism (74) and reduced NDEV Aβ42 (62). More recently, a phase IIb study in AD (n = 206) reported 12 months of treatment with liraglutide slowed cognitive decline (ADAS-EXEC) by approximately 18% and attenuated cortical atrophy by approximately 50% (140).
In summary, preclinical evidence supports a strong class effect of GLP-1RAs in AD, but clinical outcomes remain equivocal. Despite encouraging signals (stabilized glucose metabolism, reduced atrophy, favorable proteomic shifts), definitive evidence of disease modification is lacking. Larger, biomarker-driven studies will be needed and, in this regard, two large clinical trials evaluating semaglutide in early-stage symptomatic AD reported to be negative (141).
PD. Preclinical studies consistently show neuroprotective effects of GLP-1RAs in PD models. In toxin-induced rodent models of PD (MPTP, 6-OHDA, rotenone, LPS), exendin-4 (142–145), liraglutide (146, 147), lixisenatide (146), and semaglutide (57, 148) reduced dopaminergic neuron loss, preserved striatal terminals, and improved motor performance, in association with stabilization of mitochondria, reduced oxidative stress, and reduced inflammation. In more disease-relevant α-synuclein models (A53T transgenic and preformed fibril mice), PEGylated exendin-4 (NLY01, a modified form of exenatide) improved survival, motor behavior, and pathology (149). In the MitoPark mouse, one of the few preclinical models of progressive PD, a clinically translatable dose of exendin-4 reduced the rate of dopaminergic neuronal loss and accompanying motor dysfunction (150), and mitigated mitochondrial dysfunction and loss (151). In human iPSC-derived dopaminergic neurons from PD patients with SNCA mutations, exendin-4 restored insulin signaling, improved mitochondrial function, and reduced α-synuclein aggregation via PI3K/Akt/mTOR activation (26).
Epidemiological data are consistent in demonstrating that users of GLP-1RAs have reduced risk of developing PD, ranging from 25%–50% risk reduction compared with users of other oral T2DM medications (138, 152, 153). However, clinical trial results have been mixed. Two early exenatide studies suggested motor and cognitive benefits that persisted beyond drug washout (154–156), accompanied by CSF detection of the drug and enhanced brain insulin signaling (157). In contrast, subsequent larger studies utilizing NLY01 (158) failed to show benefit. Most recently, the Exenatide-PD3 phase III trial failed to show any advantage in motor or nonsymptoms over placebo (61), and questions remain about whether a subgroup of patients with PD may be more likely to respond to this therapy. More encouragingly, a phase II randomized controlled trial (RCT) of lixisenatide (n = 156) reported slower motor decline over 12 months (159), while liraglutide improved nonmotor symptoms but not motor endpoints (160).
Overall, strong preclinical data and early biomarker signals provide an encouraging rationale for GLP-1RAs in PD. In addition to neurotrophic and protective actions in dopaminergic neuron–rich brain regions and attenuation of locomotor dysfunction, amelioration of L-DOPA–induced dyskinesia has been reported across PD rodent models (161, 162). However, clinical outcomes have been inconsistent. Key priorities for future trials include selecting molecules with proven CNS penetration together with enriching for biologically plausible subgroups and incorporating biomarkers to distinguish disease-modifying effects from those confounded by weight loss, tolerability, or unblinding.
DLB. DLB shares extensive clinical and pathological overlap with PD, with both disorders characterized by α-synuclein aggregation and dopaminergic dysfunction, leading some to suggest they represent points along a single Lewy body disease spectrum (163). Importantly, BIR markers have been observed in patients with DLB, further linking disordered insulin signaling with this synucleinopathy (36). Epidemiological data in diabetic populations indicate that people taking GLP-1RAs were about 40% less likely to develop DLB, as compared with those not taking them (138). Although there are no direct models of DLB, evidence can be extrapolated from PD models and clinical studies, which (as above) demonstrate GLP-1RAs exert broad neuroprotective actions: attenuating pathological protein aggregation, improving impaired insulin signaling, and reducing neuroinflammation. These effects directly target key DLB pathologies such as widespread microglial activation observed early in the disease and may mitigate α-synuclein–mediated neurodegeneration. Although GLP-1RAs have not yet been tested directly in DLB, mixed but encouraging findings from PD trials include potential cognitive benefits (154, 155). Due to encouraging signals from PD-related studies, and in the absence of direct DLB trial data, an international Delphi consensus has prioritized GLP-1RAs as candidates for repurposing in DLBs, underscoring the need for translational studies to confirm their therapeutic efficacy (164).
MSA. As a synucleinopathy with metabolic dysfunction and rapid progression, MSA represents a logical but underexplored target for GLP-1RAs. Pathological processes such as oligodendroglial insulin signaling defects, glial inflammation, mitochondrial stress, and impaired proteostasis overlap with pathways modulated by GLP-1R stimulation.
Preclinical evidence is limited. In a PLP-SYN mouse model, high-dose exendin-4 reduced markers of BIR, lowered striatal α-synuclein burden, and preserved dopaminergic neurons, but failed to halt progressive motor decline (37).
Clinical data are also sparse. A proof-of-concept, open-label trial of weekly exenatide (2 mg, 48 weeks, 50 patients) showed an advantage in the unified multiple system atrophy rating scale (UMSARS, parts I and II combined score) at 48 weeks compared with best medically treated participants, but no difference in more objective secondary measures or biomarkers (neurofilament light chain [NfL], CSF α-synuclein oligomers) (165).
In summary, although biological plausibility is strong, evidence in MSA is restricted to one animal model and one small clinical study. Key priorities include replication in additional preclinical systems (including patient-derived oligodendrocytes), identification of molecules with reliable CNS penetration, and incorporation of biomarkers sensitive to synuclein burden and glial dysfunction. Given MSA’s rapid progression, careful patient selection and well-powered trial design will be essential to detect potential disease-modifying effects.
ALS. ALS is a relentlessly progressive disorder characterized by upper and lower motor neuron loss, excitotoxicity, and glial activation. Given that GLP-1RAs enhance insulin signaling, reduce neuroinflammation, and support mitochondrial function, they represent a plausible therapeutic strategy for ALS.
Preclinical evidence is mixed. In toxin-based models, upregulation of GLP-1/IGF-1 signaling by 4-hydroxyisoleucine improved motor behavior, preserved myelin, and reduced apoptosis (166). In SOD (G93A) ALS mice, a clinically translatable dose of exendin-4 as well as an intracerebroventricular injection of GLP-1–secreting mesenchymal stromal cells improved motor function and preserved spinal motor neurons, accompanied by reduced glial activation (167). Conversely, liraglutide failed to alter survival or pathology in SOD1G93A and TDP-43Q331K mice, underscoring uncertainty over molecule choice, dosing, and CNS penetration (168).
Human data remain limited and contradictory. In 44 patients with ALS, endogenous GLP-1 (and insulin and amylin) levels correlated with slower disease progression and inversely with the proinflammatory chemokine MCP-1, suggesting a potential protective link (169). Retrospective studies are inconsistent. One small cohort reported shorter survival in ALS patients with T2DM prescribed GLP-1RAs (170), while a large real-world dataset suggested reduced ALS risk among GLP-1RA users (138). To date, no RCTs have been conducted.
In summary, preclinical and biomarker evidence suggest GLP-1RAs may be useful in ALS, but conflicting animal and observational data highlight major uncertainties. Important next steps include exploratory early-phase trials to establish CNS penetrance, target engagement (CSF GLP-1/IGF-1), and downstream biomarkers such as MCP-1 and NfL, before larger efficacy studies can be justified.
HD. HD is an autosomal dominant disorder caused by CAG repeat expansion in the huntingtin gene (HTT), leading to progressive motor and cognitive decline with no disease-modifying treatment. Mutant huntingtin (mHTT) induces metabolic and cellular stress through impaired insulin signaling, defective autophagy, mitochondrial dysfunction, oxidative stress, and synaptic loss (171), processes that overlap with pathways modulated by GLP-1RAs.
Preclinical studies do provide early support for a protective effect of GLP-1RAs. In cell models overexpressing mHTT, liraglutide restored insulin signaling, activated AMPK, upregulated antioxidant responses, and enhanced autophagy, reducing aggregate burden and improving viability (172). In a 3-nitropropionic acid toxin model, liraglutide improved motor performance, reduced oxidative stress, and activated PI3K/Akt/CREB/BDNF signaling (173). In N171-82Q transgenic mice, exenatide improved motor function, prolonged survival, normalized glucose metabolism, and reduced mHTT aggregation in brain and pancreatic tissue (58, 174). However, evidence remains sparse, with only a few transgenic studies and no human clinical data to date. Translation will require replication in additional genetic HD models and incorporation of patient-derived iPSC systems before early clinical trials can be justified.
MS. GLP-1RAs effects are relevant to MS, with potential to modulate inflammation, promote remyelination, and to stabilize the BBB. GLP-1Rs are expressed on oligodendrocytes and Schwann cells (175), lending further support for their potential use in demyelinating disorders (175).
Preclinical studies provide mixed evidence. Exenatide improved remyelination and functional recovery in peripheral nerve injury models (176–180); however, in a cuprizone mouse model, NLY01 failed to limit demyelination or promote remyelination (181). In contrast, in experimental autoimmune encephalomyelitis (EAE) models, exendin-4, liraglutide, and dulaglutide consistently attenuate inflammation and oxidative stress (81, 87, 182), reduce clinical severity, and improve motor outcomes (83), mediated by AMPK/SIRT1 activation and NLRP3 inhibition.
Human data remain scarce. A retrospective analysis indicated GLP-1RA use was associated with reduced MS incidence (138), and a small study of dulaglutide in 13 patients demonstrated preserved endothelial function over 12 months (183). However, no trials have evaluated GLP-1RAs against standard MS clinical endpoints such as relapse rate, MRI activity, or disability progression.
Overall, GLP-1RAs reliably reduce inflammation in MS models, but effects on remyelination are inconsistent and model-dependent. Future progress will require early-phase trials using traditional MS endpoints and biomarker-driven designs.
Other neurological disorders: traumatic brain injury. Across preclinical traumatic brain injury (TBI) models (concussive, blast, fluid percussion, and controlled cortical impact), GLP-1RAs consistently mitigate neuroinflammation, oxidative stress, and stabilize the BBB, resulting in functional improvements of cognitive and motor recovery (184–186). Some effects of TBI on gene expression are reversed by clinically translatable doses of exendin-4 (187, 188); exendin-4 is also associated with inhibition of MAPK signaling that leads to suppression of proinflammatory cytokines, restoration of mitochondrial function, and improved clearance of CSF toxic metabolites through enhanced glymphatic function (189, 190). Human data are very limited, but a recent study showed that individuals with TBI had elevated GLP-1 levels, which may be compensatory and suggest dysfunctional CNS GLP-1 signaling (191). Moreover, in a recent clinical evaluation of exendin-4 in individuals with idiopathic intracranial hypertension, an immediate (within 2.5 hours) decline in intracranial pressure was reported (192), in line with the expression of GLP-1Rs at the choroid plexus and involvement in CSF generation and regulation of intracranial pressure (193). Despite promising mechanistic and physiological actions, no interventional TBI trials have yet been conducted (194).
Stroke. GLP-1RAs show robust neuroprotection in ischemic stroke models, reducing infarct size and enhancing recovery when given both before and after insult (144, 195). Meta-analyses of cardiometabolic outcome trials of GLP-1RAs demonstrate approximately 15%–20% lower stroke incidence in people with diabetes and obesity (195–197), with recent data extending benefits to nondiabetic populations (198, 199). These effects reflect indirect cardiovascular risk reduction and direct neurovascular actions, including enhanced endothelial function and reduced neuroinflammation (196, 200). However, no trials have tested GLP-1RAs as stroke treatments or secondary prevention agents, so the relative contribution of CNS versus systemic mechanisms remains unclear. Nevertheless, this building evidence has triggered calls to explore GLP-1RAs as novel treatments to reduce stroke risk and improve neurological outcomes in at-risk populations (196, 198).
Psychiatric disorders. GLP-1RAs modulate oxidative stress, inflammation, and monoaminergic and mesolimbic circuits in preclinical models, resulting in functional improvements in depression and anxiety, and also reducing drug reward (51, 201, 202). Epidemiological and genetic data are supportive. Mendelian randomization studies suggest reduced risks of schizophrenia, bipolar disorder, PTSD, and eating disorders in individuals treated with GLP-1RAs (203). A recent meta-analysis of randomized trials in obesity and/or diabetes found no increased risk of psychiatric adverse events, with improvements in quality of life, restrained eating, and emotional regulation measures (204). Thus, GLP-1RAs represent promising candidates for psychiatric disorders — particularly addiction, depression, and cognitive impairment — but dedicated clinical trials are now required to determine efficacy and define optimal therapeutic windows.
-
Challenges and opportunities in translating GLP-1RAs to neurodegeneration
Beyond GLP-1: dual and triple agonists. Since the development of single GLP-1RAs, several dual agonists (GLP-1/GIP; GLP-1/glucagon) and triple agonists (GLP-1/GIP/glucagon) are already approved or in advanced clinical development for diabetes and obesity, with translational studies now extending to neurodegeneration. These newer molecules consistently outperform single GLP-1RAs in regards to glycemic control and weight loss (205–207), although they exhibit similar gastrointestinal (GI) side effects. This superior systemic efficacy raises the possibility of stronger impact on neurodegenerative pathways that overlap with metabolic dysfunction, such as IR, mitochondrial stress, and inflammation.
Accordingly, preclinical AD and PD models suggest that dual and triple agonists may enhance neurotrophic and neuroprotective actions, while reducing Aβ and α-synuclein pathology, oxidative stress, and inflammation (49, 208, 209). Limited comparative data suggest dual and triple agonists outperform single agonists in key measures of neuroprotection and antiinflammatory response, positioning them as potentially more efficacious candidates for NDD therapy (209–213).
However, important translational challenges remain; brain penetrance varies widely between GLP-1RA–based drugs (214, 215), and evidence of the superiority of dual and triple agonists in modulating neurodegenerative pathways remains confined to cell and animal models. Whether these benefits translate into meaningful clinical efficacy in human NDDs remains unknown. A systematic translational roadmap will be required to realize their potential in NDDs. This would include comparative preclinical studies using standardized models of proteinopathy and metabolic dysfunction to define class-specific mechanisms, followed by early-phase human trials incorporating pharmacokinetic and biomarker endpoints of brain target engagement. Such work will clarify whether enhanced metabolic and antiinflammatory actions translate into measurable clinical benefit.
CNS delivery and target engagement. GLP-1RAs represent a pharmacologically diverse class, differing in receptor kinetics, molecular size, half-life, and capacity to cross the BBB, which likely contributes to variability in neuroprotective efficacy across compounds. Radiolabel studies suggest small peptides like exenatide and lixisenatide cross the BBB, while acylated or PEGylated analogues (liraglutide, semaglutide, NLY01) show minimal uptake. The clinical failure of NLY01 in PD, despite evidence of CNS penetration in nonhuman primates (158), underscores the uncertainty around how brain access relates to clinical efficacy.
Human trial data are inconsistent but suggest variability between drugs and formulations: exenatide (Bydureon) reached 1%–3% of peripheral levels in CSF (156), while another formulation of once-weekly exentide (Bcise) only achieved approximately 1% of peripheral levels detectable in CSF (61). While liraglutide does not seem to cross the BBB (216), improvements have been reported in AD and PD cohorts (97, 217).
A key unresolved issue is whether direct BBB penetration is required or whether therapeutic effects can arise from circumventricular activation, peripheral metabolic improvement, or systemic immunomodulation. Recent data also suggest that some antiinflammatory effects of GLP-1RAs may be centrally mediated even with minimal penetration (218). Adding further complexity, GLP-1RAs can stabilize the BBB (219, 220), raising the possibility that drugs with limited baseline uptake may improve their own CNS access over time, particularly in aging or NDD states where BBB dysfunction is common. It remains unclear whether GLP-1RAs primarily exploit disease-related BBB dysfunction or actively remodel the barrier to support neuroprotection.
Safety, adherence, and patient stratification. Although generally safe, GLP-1RAs cause consistent GI side effects that contribute to dose interruptions and discontinuations in obesity and diabetes trials. In older or frail populations, concerns include lean-mass loss, aspiration risk during procedures, sarcopenia, falls, and worsening dysphagia. Because weight loss is already common across NDDs, excessive or rapid GLP-1RA–induced weight loss may exacerbate frailty, potentially offsetting neurological benefits. Careful monitoring of nutrition and lean mass should therefore be integrated into NDD trials.
Beyond tolerability, adherence strongly influences outcomes. Real-world discontinuation rates for GLP-1RAs range from 17%–35% in the first year, varying by drug and country (221). These challenges are likely to be greater in older adults with cognitive or motor decline. Trials will therefore require structured support — slow titration, proactive side-effect management, nutritional and exercise guidance, and caregiver involvement — to prevent dilution of efficacy signals.
Even when treatment is continued, there remains great heterogeneity of response, exemplified by only approximately 50%–70% of patients achieving clinically meaningful weight loss outside of clinical trials (221, 222). Response rates vary by molecule — newer agents such as tirzepatide and semaglutide yield higher responder rates (~86%–91%) than older agents such as exenatide or liraglutide (~40%–65%) (223) — and vary by baseline BMI, sex, and glycemic status, with women and individuals with higher BMI responding better (222, 224). Genetic and pharmacogenomic studies implicate GLP1R, ARRB1 (β-arrestin-1), and other loci in contributing to interindividual variability (225).
Despite years of clinical experience in diabetes and obesity, predicting responders to GLP-1RAs remains imprecise, closer to “tossing a coin” than true precision medicine (224, 226). Early exploratory work in NDDs suggests that individuals with evidence of brain or IR may benefit most (26), and post hoc analyses hint that higher BMI, younger age, T2DM comorbidity, and earlier disease stage might predict better response (227). Future NDD trials will likely need biological enrichment strategies (e.g., selecting BIR-positive or metabolically dysregulated individuals), combined with biomarker endpoints of central insulin signaling and stringent adherence frameworks, to reliably identify responders.
Broader translational hurdles: outcome measures and trial design. Traditional clinical endpoints often lack sensitivity to detect disease-modifying effects, particularly over short trial durations. Weight loss and GI side effects risk unblinding, potentially inflating placebo responses. Innovative designs incorporating active comparators, adaptive frameworks, and biomarker-driven endpoints (e.g., CSF/serum NfL, p-tau, microglial PET, synaptic markers) will be critical to generate interpretable efficacy signals.
Timing of intervention. In preclinical studies, GLP-1RAs are typically administered before or immediately after the onset of pathology, whereas clinical trials have enrolled mild-to-moderate symptomatic cohorts. Earlier intervention — at prodromal or preclinical stages — is likely to enhance efficacy. Future trials should include stage-stratified enrollment. Whether GLP-1RAs can modify disease once substantial neuronal loss has occurred remains uncertain, although benefits on later features (e.g., cognitive decline, gait or balance impairments) merit evaluation.
Combination approaches and disease heterogeneity. With anti-Aβ and anti-tau therapies entering AD practice, GLP-1RAs may be most effective in combination, targeting metabolic and inflammatory pathways that complement direct amyloid or tau lowering. Similarly, GLP-1RAs may offer greatest benefit in disorders with strong metabolic and proteinopathy links (AD, PD, MS), whereas effects in primarily RNA-driven diseases such as ALS or HD may be more limited. Disease-specific prioritization will be essential.
Practical and economic considerations. Injectable formulations may pose barriers for frail or cognitively impaired patients, and the demand for GLP-1RAs in obesity and diabetes creates challenges for scalable use in NDDs. Development of oral, depot, or transdermal delivery systems may improve adherence. Cost effectiveness and equitable access will need careful consideration if GLP-1RAs are to be deployed more broadly in NDDs. Emerging oral and long-acting formulations may overcome barriers to self-injection in frail or cognitively impaired populations.
-
Conclusions
Across preclinical and translational studies, GLP-1RAs have emerged as promising candidates for NDD therapy, consistently modulating convergent mechanisms of pathology: IR, mitochondrial dysfunction, oxidative stress, proteostasis failure, synaptic loss, and neuroinflammation. Evidence for their potential application in AD and PD is strongest, with signals of target engagement in humans, while data in rarer disorders such as MSA, ALS, HD, and MS remain sparse and inconsistent. Observational studies suggest potential protective associations, but clinical trials to date show mixed efficacy, highlighting unresolved challenges around molecule selection, CNS penetration, tolerability, adherence, and patient stratification.
Progress will depend on biomarker-driven, biologically enriched trial designs capable of determining whether the pleiotropic benefits observed in experimental systems translate into meaningful disease modification.
A useful conceptual framework comes from exercise, one of the few interventions with proven disease-modifying influence in NDDs. Structured physical activity enhances vascular and metabolic health, increases neurotrophic signaling, strengthens synaptic plasticity, and bolsters mitochondrial resilience, with benefits documented across aging, AD, and PD populations (228–230). These pleiotropic actions mirror many attributed to GLP-1RAs — enhancing insulin signaling, reducing neuroinflammation, and stabilizing energy homeostasis. This parallel suggests a unifying therapeutic principle: GLP-1RAs may act as a “pharmacological analogue of exercise,” offering a complementary strategy where lifestyle interventions alone are insufficient or impractical. Framed in this way, the rationale for their continued development as disease-modifying therapies in NDDs is strong, but must now be matched by well-designed, biomarker-rich clinical trials.
-
Funding support
This work is the result of NIH funding, in whole or in part, and is subject to the NIH Public Access Policy. Through acceptance of this federal funding, the NIH has been given a right to make the work publicly available in PubMed Central.
- Medical Research Council/National Institute for Health and Care Research (NIHR) Clinical Academic Research Partnership (to DA).
- Cure Parkinson’s Trust (to DA and TF).
- NIHR (to TF).
- MJFox Foundation (to TF and SG).
- John Black Charitable Foundation (to TF).
- Janet Owens Research fellowship (to TF).
- Edmond J Safra Foundation (to TF).
- Van Andel Institute (to TF).
- Defeat MSA (to TF).
- UCLH Biomedical Research Centre (TF and SG).
- MRC Senior Clinical Fellow, MR/T008199/1 (SG).
-
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the NIH. The contributions of NHG are considered works of the US government. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Address correspondence to: Dilan Athauda, The Francis Crick Institute, 1 Midland Road, London NW1 1AT, United Kingdom. Email: dilan.athauda@crick.ac.uk.
-
Footnotes
Conflict of interest: TF has served on advisory boards for Peptron, Abbvie, Eli Lilly, Bluerock, Bayer, and Bial. He has received honoraria for talks sponsored by Bayer, Bial, Boston Scientific, and Novo Nordisk. Outside the present manuscript, WGM has received consultancy fees from Lundbeck, Inhibikase, Koneksa, Ferrer, TreeFrog, Takeda, and BioArctic. He has also received fees for lecturing from Lundbeck.
Copyright: © 2026, Athauda et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2026;136(4):e194745. https://doi.org/10.1172/JCI194745.
-
References
- Su D, et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: modelling study of Global Burden of Disease Study 2021. BMJ. 2025;388:e080952.
- Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl). 2004;82(8):510–529.
- Athanasaki A, et al. Type 2 diabetes mellitus as a risk factor for Alzheimer’s disease: review and meta-analysis. Biomedicines. 2022;10(4):778.
- Pagano G, et al. Age at onset and Parkinson disease phenotype. Neurology. 2016;86(15):1400–1407.
- De Pablo-Fernandez E, et al. Association between Parkinson’s disease and diabetes: data from NEDICES study. Acta Neurol Scand. 2017;136(6):732–736.
- Han K, et al. A nationwide cohort study on diabetes severity and risk of Parkinson disease. NPJ Parkinsons Dis. 2023;9(1):11.
- Hou W-H, et al. A population-based cohort study suggests an increased risk of multiple sclerosis incidence in patients with type 2 diabetes mellitus. J Epidemiol. 2017;27(5):235–241.
- Martinelli I, et al. Exploring the role of diabetes in ALS: a population-based cohort study. Life (Basel). 2025;15(6):936. View this article via: PubMed Google Scholar
- Skajaa N, et al. Type 2 diabetes, obesity, and risk of amyotrophic lateral sclerosis: a population-based cohort study. Brain Behav. 2023;13(6):e3007.
- Zhang L, et al. Association between type 2 diabetes and amyotrophic lateral sclerosis. Sci Rep. 2022;12(1):2544.
- Yeh T-S, et al. Type 2 diabetes mellitus, antidiabetics, and the risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2025;26(7-8):775–783.
- Zeidan O, et al. The influence of insulin resistance and type 2 diabetes on cognitive decline and dementia in Parkinson’s disease: a systematic review. Int J Mol Sci. 2025;26(16):8078.
- Willette AA, et al. Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes. 2015;64(6):1933–1940.
- Pagano G, et al. Diabetes mellitus and Parkinson disease. Neurology. 2018;90(19):1654–1662.
- Athauda D, et al. The impact of type 2 diabetes in parkinson’s disease. Mov Disord. 2022;37(8):1612–1623.
- Spinelli M, et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat Commun. 2017;8(1):2009.
- Spinelli M, et al. Brain insulin resistance and hippocampal plasticity: mechanisms and biomarkers of cognitive decline. Front Neurosci. 2019;13:788.
- Rodriguez-Rodriguez P, et al. Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain. 2017;140(12):3269–3285.
- Chatterjee S, et al. Insulin-mediated changes in tau hyperphosphorylation and autophagy in a drosophila model of tauopathy and neuroblastoma cells. Front Neurosci. 2019;13:801.
- Gonçalves RA, et al. The link between tau and insulin signaling: implications for Alzheimer’s disease and other tauopathies. Front Cell Neurosci. 2019;13:17.
- Leboucher A, et al. Brain insulin response and peripheral metabolic changes in a Tau transgenic mouse model. Neurobiol Dis. 2019;125:14–22.
- Wei Z, et al. Insulin resistance exacerbates Alzheimer disease via multiple mechanisms. Front Neurosci. 2021;15:687157.
- Tian Y, et al. Insulin-degrading enzyme: roles and pathways in ameliorating cognitive impairment associated with Alzheimer’s disease and diabetes. Ageing Res Rev. 2023;90:101999.
- Zhou AL, et al. Blood-brain barrier insulin resistance decreases insulin uptake and increases amyloid beta uptake in Alzheimer’s disease brain. Alzheimers Dement. 2020;16(s3):e047353. View this article via: CrossRef Google Scholar
- Gao S, et al. Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int J Biochem Cell Biol. 2015;64:25–33.
- Athauda D, et al. GLP1 receptor agonism ameliorates Parkinson’s disease through modulation of neuronal insulin signalling and glial suppression [preprint]. https://doi.org/10.1101/2024.02.28.582460 Posted on bioRxiv February 28, 2024.
- Ponce-Lopez T. Peripheral inflammation and insulin resistance: their impact on blood-brain barrier integrity and glia activation in Alzheimer’s disease. Int J Mol Sci. 2025;26(9):4209.
- Darwish R, et al. The role of hypothalamic microglia in the onset of insulin resistance and type 2 diabetes: a neuro-immune perspective. Int J Mol Sci. 2024;25(23):13169.
- Yoon JH, et al. How can insulin resistance cause Alzheimer’s disease? Int J Mol Sci. 2023;24(4):3506.
- Ntetsika T, et al. Understanding the link between type 2 diabetes mellitus and Parkinson’s disease: role of brain insulin resistance. Neural Regen Res. 2024;20(11):3113–3123.
- Zhao W-Q, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22(1):246–260.
- Xie L, et al. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22(10):RC221.
- Santiago JA, et al. Diabetes: a tipping point in neurodegenerative diseases. Trends Mol Med. 2023;29(12):1029–1044.
- Bassil F, et al. Brain insulin resistance in Parkinson’s disease. Mov Disord. 2017;32(suppl 2):526. View this article via: PubMed Google Scholar
- Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338.
- Yarchoan M, et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128(5):679–689.
- Bassil F, et al. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017;140(5):1420–1436.
- Chung SJ, et al. Detrimental effect of type 2 diabetes mellitus in a large case series of Parkinson’s disease. Parkinsonism Relat Disord. 2019;64:54–59.
- Petrou M, et al. Diabetes, gray matter loss, and cognition in the setting of Parkinson disease. Acad Radiol. 2016;23(5):577–581.
- Bohnen NI, et al. Diabetes mellitus is independently associated with more severe cognitive impairment in Parkinson disease. Parkinsonism Relat Disord. 2014;20(12):1394–1398.
- Baker LD, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68(1):51–57.
- Andreassen OA, et al. Huntington’s disease of the endocrine pancreas: insulin deficiency and diabetes mellitus due to impaired insulin gene expression. Neurobiol Dis. 2002;11(3):410–424.
- López-Mora DA, et al. Striatal hypometabolism in premanifest and manifest Huntington’s disease patients. Eur J Nucl Med Mol Imaging. 2016;43(12):2183–2189.
- Sepidarkish M, et al. Association between insulin resistance and multiple sclerosis: a systematic review and meta-analysis. Metab Brain Dis. 2024;39(5):1015–1026.
- Daniels D, Mietlicki-Baase EG. Glucagon-like peptide 1 in the brain: where is it coming from, where is it going? Diabetes. 2019;68(1):15–17.
- Roy A, et al. From metabolism to mind: the expanding role of the GLP-1 receptor in neurotherapeutics. Neurotherapeutics. 2025;22(5):e00712.
- Alvarez E, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92(4):798–806.
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21(5):802–818.
- Reich N, Hölscher C. The neuroprotective effects of glucagon-like peptide 1 in Alzheimer’s and Parkinson’s disease: an in-depth review. Front Neurosci. 2022;16:970925.
- Zhou ZD, et al. Glucagon-like peptide-1 receptor agonists in neurodegenerative diseases: promises and challenges. Pharmacol Res. 2025;216:107770.
- Giorgi RD, et al. Glucagon-like peptide-1 receptor agonists for major neurocognitive disorders. J Neurol Neurosurg Psychiatry. 2025;96(9):870–883.
- Nowell J, et al. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol Psychiatry. 2023;28(1):217–229.
- Diz-Chaves Y, et al. Anti-inflammatory effects of GLP-1 receptor activation in the brain in neurodegenerative diseases. Int J Mol Sci. 2022;23(17):9583.
- Zhou M, et al. Dulaglutide ameliorates STZ induced AD-like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3β. Biochem Biophys Res Commun. 2019;511(1):154–160.
- Ma DL, et al. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J Neurochem. 2015;135(2):301–308.
- Batista AF, et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol. 2018;245(1):85–100.
- Zhang L, et al. Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of Parkinson’s disease. J Parkinsons Dis. 2019;9(1):157–171.
- Martin B, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington’s disease. Diabetes. 2009;58(2):318–328.
- Li Y, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7(2):e32008.
- Athauda D, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–1675.
- Vijiaratnam N, et al. Exenatide once a week versus placebo as a potential disease-modifying treatment for people with Parkinson’s disease in the UK: a phase 3, multicentre, double-blind, parallel-group, randomised, placebo-controlled trial. Lancet. 2025;405(10479):627–636.
- Mullins RJ, et al. A pilot study of exenatide actions in Alzheimer’s disease. Curr Alzheimer Res. 2019;16(8):741–752.
- Henrich MT, et al. Mitochondrial dysfunction in Parkinson’s disease - a key disease hallmark with therapeutic potential. Mol Neurodegener. 2023;18(1):83.
- Minoshima S, et al. 18F-FDG PET imaging in neurodegenerative dementing disorders: insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. J Nucl Med. 2022;63(suppl 1):2S–12S.
- Vercruysse P, et al. Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front Mol Neurosci. 2018;11:2.
- Signorile A, et al. cAMP/PKA signaling modulates mitochondrial supercomplex organization. Int J Mol Sci. 2022;23(17):9655.
- Spry ER, et al. The effect of semaglutide on mitochondrial function and insulin sensitivity in a myotube model of insulin resistance. Mol Cell Endocrinol. 2025;608:112629.
- Zhou Y, et al. SIRT1/PGC-1α signaling promotes mitochondrial functional recovery and reduces apoptosis after intracerebral hemorrhage in rats. Front Mol Neurosci. 2018;10:443.
- Soutar MPM, et al. AKT signalling selectively regulates PINK1 mitophagy in SHSY5Y cells and human iPSC-derived neurons. Sci Rep. 2018;8(1):8855.
- Chen K, et al. Glucagon-like peptide-1 receptor agonist exendin 4 ameliorates diabetes-associated vascular calcification by regulating mitophagy through the AMPK signaling pathway. Mol Med. 2024;30(1):58.
- Hu X, et al. Important regulatory role of mitophagy in diabetic microvascular complications. J Transl Med. 2025;23(1):269.
- Zheng J, et al. GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer’s disease. Mol Metab. 2021;47:101180.
- Gejl M, et al. Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci Rep. 2017;7(1):17490.
- Gejl M, et al. In Alzheimer’s disease, six-month treatment with GLP-1 analogue prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108.
- Yang JL, et al. Activation of GLP-1 receptor enhances neuronal base excision repair via PI3K-AKT-induced expression of apurinic/apyrimidinic endonuclease 1. Theranostics. 2016;6(12):2015–2027.
- Yang JL, et al. The emerging role of GLP-1 receptors in DNA repair: implications in neurological disorders. Int J Mol Sci. 2017;18(9):1861.
- Timper K, et al. GLP-1 receptor signaling in astrocytes regulates fatty acid oxidation, mitochondrial integrity, and function. Cell Metab. 2020;31(6):1189–1205.
- Qian Z, et al. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury. Int J Biol Sci. 2022;18(4):1328–1346.
- Diz-Chaves Y, et al. Glucagon-like peptide 1 receptor activation: anti-inflammatory effects in the brain. Neural Regen Res. 2023;19(8):1671–1677.
- Muraleedharan R, Dasgupta B. AMPK in the brain: its roles in glucose and neural metabolism. FEBS J. 2022;289(8):2247–2262.
- Chiou HYC, et al. Dulaglutide modulates the development of tissue-infiltrating Th1/Th17 cells and the pathogenicity of encephalitogenic Th1 cells in the central nervous system. Int J Mol Sci. 2019;20(7):1584.
- Xu W, et al. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol Immunol. 2025;22(4):341–355.
- Ammar RA, et al. Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: role of AMPK/SIRT1 signaling and NLRP3 inflammasome. Inflammopharmacology. 2022;30(3):919–934.
- Gulen MF, et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620(7973):374–380.
- Huang Y, et al. Mechanism and therapeutic potential of targeting cGAS-STING signaling in neurological disorders. Mol Neurodegener. 2023;18(1):79.
- Kim S, et al. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol. 2009;202(3):431–439.
- DellaValle B, et al. Glucagon-like peptide-1 analog, liraglutide, delays onset of experimental autoimmune encephalitis in Lewis rats. Front Pharmacol. 2016;7:433.
- Koychev I, et al. Inflammatory proteins associated with Alzheimer’s disease reduced by a GLP1 receptor agonist: a post hoc analysis of the EXSCEL randomized placebo controlled trial. Alzheimers Res Ther. 2024;16(1):212.
- Wang Q, et al. Comparison between the effects of sitagliptin and liraglutide on blood glucose and cognitive function of patients with both type 2 diabetes mellitus and post-stroke mild cognitive impairment. Int J Clin Exp Med. 2020;13(2):1219–1227.
- Ma Q, et al. GLP-1 plays a protective role in hippocampal neuronal cells by activating cAMP-CREB-BDNF signaling pathway against CORT+HG-induced toxicity. Heliyon. 2023;9(8):e18491.
- Du H, et al. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front Endocrinol (Lausanne). 2022;13:1033479.
- Zheng Z, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):234.
- Urkon M, et al. Antidiabetic GLP-1 receptor agonists have neuroprotective properties in experimental animal models of Alzheimer’s disease. Pharmaceuticals (Basel). 2025;18(5):614.
- Li Y, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1205–1219.
- Lin CL, et al. Stimulation of GLP-1 signaling enhances protein clearance and reduces Aβ-mediated toxicity in neuronal cells. Alzheimers Dement. 2020;16(s3):e038285. View this article via: CrossRef Google Scholar
- Wang M, et al. Exendin-4 improves long-term potentiation and neuronal dendritic growth in vivo and in vitro obesity condition. Sci Rep. 2021;11(1):8326.
- Alzheimer’s Association. Research Advances at the Alzheimer’s Association International Conference. https://aaic.alz.org/releases-2024/highlights-aaic-2024.asp Published August 1, 2024. Accessed August 30, 2025.
- Loh JS, et al. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024;9(1):37.
- Park KJ, Gao Y. Gut-brain axis and neurodegeneration: mechanisms and therapeutic potentials. Front Neurosci. 2024;18:1481390.
- Yuan C, et al. Review of microbiota gut brain axis and innate immunity in inflammatory and infective diseases. Front Cell Infect Microbiol. 2023;13:1282431.
- O’Riordan KJ, et al. The gut microbiota-immune-brain axis: therapeutic implications. Cell Rep Med. 2025;6(3):101982.
- Elkins M, et al. The menace within: bacterial amyloids as a trigger for autoimmune and neurodegenerative diseases. Curr Opin Microbiol. 2024;79:102473.
- Ghosh N, Sinha K. Multi-ancestry GWAS reveals loci linked to human variation in LINE-1- and Alu-insertion numbers. Transl Med Aging. 2025;9:25–40.
- Sun J, et al. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun. 2021;91:703–715.
- Abdalqadir N, Adeli K. GLP-1 and GLP-2 orchestrate intestine integrity, gut microbiota, and immune system crosstalk. Microorganisms. 2022;10(10):2061.
- Zhang Q, et al. Featured article: Structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Exp Biol Med (Maywood). 2018;243(1):34–44.
- Kant R, et al. Gut microbiota interactions with anti-diabetic medications and pathogenesis of type 2 diabetes mellitus. World J Methodol. 2022;12(4):246–257.
- Nowakowska J, et al. Differences in gut microbiota modulation by semaglutide, liraglutide and tirzepatide. Int J Innov Technol Soc Sci. 2025;23(47):3563.
- Chen J, et al. GLP-1 receptor agonist as a modulator of innate immunity. Front Immunol. 2022;13:997578.
- Li X, et al. GLP-1RAs inhibit the activation of the NLRP3 inflammasome signaling pathway to regulate mouse renal podocyte pyroptosis. Acta Diabetol. 2024;61(2):225–234.
- Ma LY, et al. Liraglutide improves cognition function in streptozotocin-induced diabetic rats by downregulating β-secretase and γ-secretase and alleviating oxidative stress in HT-22 cells. Endocr J. 2025;72(3):285–294.
- Xiong H, et al. The neuroprotection of liraglutide on Alzheimer-like learning and memory impairment by modulating the hyperphosphorylation of tau and neurofilament proteins and insulin signaling pathways in mice. J Alzheimers Dis. 2013;37(3):623–635.
- Wang X, et al. Exendin-4 antagonizes Aβ1-42-induced attenuation of spatial learning and memory ability. Exp Ther Med. 2016;12(5):2885–2892.
- Qi L, et al. Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced alzheimer disease mouse model. Eur J Pharmacol. 2016;783:23–32.
- Jia XT, et al. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects against amyloid-β peptide-induced impairment of spatial learning and memory in rats. Physiol Behav. 2016;159:72–79.
- Han WN, et al. Liraglutide protects against amyloid-β protein-induced impairment of spatial learning and memory in rats. Neurobiol Aging. 2013;34(2):576–588.
- Garabadu D, Verma J. Exendin-4 attenuates brain mitochondrial toxicity through PI3K/Akt-dependent pathway in amyloid beta (1-42)-induced cognitive deficit rats. Neurochem Int. 2019;128(1-42):39–49.
- Cai HY, et al. Lixisenatide rescues spatial memory and synaptic plasticity from amyloid β protein-induced impairments in rats. Neuroscience. 2014;277:6–13.
- Perry T, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72(5):603–612.
- Perry T, et al. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302(3):881–888.
- An J, et al. Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5×FAD mouse model of Alzheimer’s disease. Behav Brain Res. 2019;370:111932.
- Wang Y, et al. GLP-1 receptor agonists downregulate aberrant GnT-III expression in Alzheimer’s disease models through the Akt/GSK-3β/β-catenin signaling. Neuropharmacology. 2018;131:190–199.
- Elbadawy NN, et al. The GLP-1 agonist semaglutide ameliorates cognitive regression in P301S tauopathy mice model via autophagy/ACE2/SIRT1/FOXO1-mediated microglia polarization. Eur J Pharmacol. 2025;991:177305.
- Zhai Y, et al. Semaglutide improves cognitive function and neuroinflammation in APP/PS1 transgenic mice by activating AMPK and inhibiting TLR4/NF-κB pathway. J Alzheimers Dis. 2025;105(2):416–432.
- McClean PL, et al. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31(17):6587–6594.
- McClean PL, Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76 Pt A:57–67.View this article via: PubMed Google Scholar
- Zhang M, et al. Glucagon-like peptide-1 analogs mitigate neuroinflammation in Alzheimer’s disease by suppressing NLRP2 activation in astrocytes. Mol Cell Endocrinol. 2022;542:111529.
- Robinson A, et al. Combination of insulin with a GLP1 agonist is associated with better memory and normal expression of insulin receptor pathway genes in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2019;67(4):504–510.
- McClean PL, et al. Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation and memory impairment in APP/PS1 mice. Behav Brain Res. 2015;293:96–106.
- Hansen HH, et al. Long-term treatment with liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has no effect on β-amyloid plaque load in two transgenic APP/PS1 mouse models of Alzheimer’s disease. PLoS One. 2016;11(7):e0158205.
- Chen S, et al. Liraglutide improves water maze learning and memory performance while reduces hyperphosphorylation of tau and neurofilaments in APP/PS1/tau triple transgenic mice. Neurochem Res. 2017;42(8):2326–2335.
- Bomfim TR, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J Clin Invest. 2012;122(4):1339–1353.
- Bomba M, et al. Exenatide reverts the high-fat-diet-induced impairment of BDNF signaling and inflammatory response in an animal model of Alzheimer’s disease. J Alzheimers Dis. 2019;70(3):793–810.
- Zhang Y, et al. Semaglutide ameliorates Alzheimer’s disease and restores oxytocin in APP/PS1 mice and human brain organoid models. Biomed Pharmacother. 2024;180:117540.
- Forny Germano L, et al. The GLP-1 medicines semaglutide and tirzepatide do not alter disease-related pathology, behaviour or cognitive function in 5XFAD and APP/PS1 mice. Mol Metab. 2024;89:102019.
- Vogrinc D, et al. Genetic variability of incretin receptors affects the occurrence of neurodegenerative diseases and their characteristics. Heliyon. 2024;10(20):e39157.
- Seminer A, et al. Cardioprotective glucose-lowering agents and dementia risk: a systematic review and meta-analysis. JAMA Neurol. 2025;82(5):450–460.
- Siddeeque N, et al. Evaluating the therapeutic potential of GLP-1 agonists in neurodegenerative disorders (S25.006). Neurology. 2025;104(7_suppl_1):1616. View this article via: CrossRef Google Scholar
- Maretty L, et al. Proteomic changes upon treatment with semaglutide in individuals with obesity. Nat Med. 2025;31(1):267–277.
- Edison P, et al. Liraglutide in mild to moderate Alzheimer’s disease: a phase 2b clinical trial. [published online December 1, 2025]. Nat Med. https://doi.org/10.1038/s41591-025-04106-7. View this article via: PubMed Google Scholar
- Cummings JL, et al. evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimers Res Ther. 2025;17(1):14.
- Bertilsson G, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86(2):326–338.View this article via: CrossRef Google Scholar
- Harkavyi A, Whitton PS. Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br J Pharmacol. 2010;159(3):495–501.
- Li Y, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–1290.
- Aksoy D, et al. Neuroprotective effects of eexenatide in a rotenone-induced rat model of Parkinson’s disease. Am J Med Sci. 2017;354(3):319–324.
- Liu W, et al. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50.
- Badawi GA, et al. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson’s disease. Inflammopharmacology. 2017;25(3):369–382.
- Zhang L, et al. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides. 2018;71:70–80.
- Park J-S, et al. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9(1):78.
- Wang V, et al. Sustained release GLP-1 agonist PT320 delays disease progression in a mouse model of Parkinson’s disease. ACS Pharmacol Transl Sci. 2021;4(2):858–869.
- Wang V, et al. Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice. J Biomed Sci. 2024;31(1):38.
- Brauer R, et al. Diabetes medications and risk of Parkinson’s disease: a cohort study of patients with diabetes. Brain. 2020;143(10):3067–3076.
- Tang H, et al. Glucagon-like peptide-1 receptor agonists and risk of Parkinson’s disease in patients with type 2 diabetes: a population-based cohort Study. Mov Disord. 2024;39(11):1960–1970.
- Aviles-Olmos I, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730–2736.
- Aviles-Olmos I, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337–344.View this article via: CrossRef Google Scholar
- Athauda D, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–1675.
- Athauda D, et al. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol. 2019;76(4):420–429.
- McGarry A, et al. Safety, tolerability, and efficacy of NLY01 in early untreated Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2024;23(1):37–45.
- Meissner WG, et al. Trial of lixisenatide in early Parkinson’s disease. N Engl J Med. 2024;390(13):1176–1185.
- Malatt C, et al. Liraglutide improves non-motor function and activities of daily living in patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial (P9-11.005). Neurology. 2022;98(18_supplement):3068. View this article via: CrossRef Google Scholar
- Badawi GA, et al. Sitagliptin and liraglutide modulate l-dopa effect and attenuate dyskinetic movements in rotenone-lesioned rats. Neurotox Res. 2019;35(3):635–653.
- Kuo T-T, et al. PT320, a sustained-release GLP-1 receptor agonist, ameliorates L-DOPA-induced dyskinesia in a mouse model of Parkinson’s disease. Int J Mol Sci. 2023;24(5):4687.
- Borghammer P, et al. Parkinson’s disease and dementia with Lewy bodies: one and the same. J Parkinsons Dis. 2024;14(3):383–397.
- O’Brien JT, et al. RENEWAL: REpurposing study to find NEW compounds with Activity for Lewy body dementia-an international Delphi consensus. Alzheimers Res Ther. 2022;14(1):169.
- Vijiaratnam N, et al. Exenatide once weekly in the treatment of patients with multiple system atrophy. Ann Neurol. 2025;98(5):991–1003.
- Shandilya A, et al. Activation of IGF-1/GLP-1 signalling via 4-hydroxyisoleucine prevents motor neuron impairments in experimental ALS-rats exposed to methylmercury-induced neurotoxicity. Molecules. 2022;27(12):3878.
- Knippenberg S, et al. Intracerebroventricular injection of encapsulated human mesenchymal cells producing glucagon-like peptide 1 prolongs survival in a mouse model of ALS. PLoS One. 2012;7(6):e36857.
- Keerie A, et al. The GLP-1 receptor agonist, liraglutide, fails to slow disease progression in SOD1G93A and TDP-43Q331K transgenic mouse models of ALS. Sci Rep. 2021;11(1):17027.
- Moțățăianu A, et al. Exploring the role of metabolic hormones in amyotrophic lateral sclerosis. Int J Mol Sci. 2024;25(10):5059.
- Lee I, et al. GLP-1-related antihyperglycemic medication use is associated with shorter survival in patients with amyotrophic lateral sclerosis and diabetes mellitus (P11-11.032). Neurology. 2025;104(7_suppl_1):2223. View this article via: CrossRef Google Scholar
- Franco-Iborra S, et al. Mutant HTT (huntingtin) impairs mitophagy in a cellular model of Huntington disease. Autophagy. 2021;17(3):672–689.
- Chang CC, et al. GLP-1 analogue liraglutide attenuates mutant huntingtin-induced neurotoxicity by restoration of neuronal insulin signaling. Int J Mol Sci. 2018;19(9):2505.
- Shawki SM, et al. Liraglutide improves cognitive and neuronal function in 3-NP rat model of Huntington’s disease. Front Pharmacol. 2021;12:731483.
- Martin B, et al. Euglycemic agent-mediated hypothalamic transcriptomic manipulation in the N171-82Q model of Huntington disease is related to their physiological efficacy. J Biol Chem. 2012;287(38):31766–31782.
- Sango K, et al. Glucagon-like peptide-1 receptor agonists as potential myelination-inducible and anti-demyelinating remedies. Front Cell Dev Biol. 2022;10:950623.
- Kuyucu E, et al. Exenatide promotes regeneration of injured rat sciatic nerve. Neural Regen Res. 2017;12(4):637–643.
- Takaku S, et al. Exendin-4 promotes Schwann cell survival/migration and myelination in vitro. Int J Mol Sci. 2021;22(6):2971.
- Yamamoto K, et al. Therapeutic effect of exendin-4, a long-acting analogue of glucagon-like peptide-1 receptor agonist, on nerve regeneration after the crush nerve injury. Biomed Res Int. 2013;2013:315848.
- Perry T, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203(2):293–301.
- de Sousa E, et al. Incretin receptors in the peripheral nervous system: implications for obesity treatment and peripheral neuropathy. Diabetes. 2025;74(8):1313–1319.
- Gharagozloo M, et al. The effects of NLY01, a novel glucagon-like peptide-1 receptor agonist, on cuprizone-induced demyelination and remyelination: challenges and future perspectives. Neurotherapeutics. 2023;20(4):1229–1240.
- Lee CH, et al. Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol Neurobiol. 2018;55(4):3007–3020.
- Hardonova M, et al. Endothelial function in patients with multiple sclerosis: the role of GLP-1 agonists, lipoprotein subfractions, and redox balance. Int J Mol Sci. 2023;24(13):11162.
- Eakin K, et al. Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS One. 2013;8(12):e82016.
- Zhang J, et al. Glucagon-like peptide-1 receptor agonist Exendin-4 improves neurological outcomes by attenuating TBI-induced inflammatory responses and MAPK activation in rats. Int Immunopharmacol. 2020;86:106715.
- Li Y, et al. Neurotrophic and neuroprotective effects of a monomeric GLP-1/GIP/Gcg receptor triagonist in cellular and rodent models of mild traumatic brain injury. Exp Neurol. 2020;324:113113.
- Tweedie D, et al. Blast traumatic brain injury-induced cognitive deficits are attenuated by preinjury or postinjury treatment with the glucagon-like peptide-1 receptor agonist, exendin-4. Alzheimers Dement. 2016;12(1):34–48.
- Tweedie D, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013;239:170–182.
- Lv C, et al. Cerebral glucagon-like peptide-1 receptor activation alleviates traumatic brain injury by glymphatic system regulation in mice. CNS Neurosci Ther. 2023;29(12):3876–3888.
- Glotfelty EJ, et al. Incretin mimetics as rational candidates for the treatment of traumatic brain injury. ACS Pharmacol Transl Sci. 2019;2(2):66–91.
- Zhou M, et al. Central GLP-1 resistance induced by severe traumatic brain injury was associated with persistent hyperglycemia in humans. Neuroendocrinology. 2023;113(6):625–640.
- Mitchell JL, et al. The effect of GLP-1RA exenatide on idiopathic intracranial hypertension: a randomized clinical trial. Brain. 2023;146(5):1821–1830.
- Botfield HF, et al. A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med. 2017;9(404):eaan0972.
- Harej Hrkać A, et al. The therapeutic potential of glucagon-like peptide 1 receptor agonists in traumatic brain injury. Pharmaceuticals (Basel). 2024;17(10):1313.
- Wei J, et al. Risk of stroke and retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: an eight RCTs meta-analysis. Front Endocrinol (Lausanne). 2022;13:1007980.
- Goldenberg RM, et al. Benefits of GLP-1 (glucagon-like peptide 1) receptor agonists for stroke reduction in type 2 diabetes: a call to action for neurologists. Stroke. 2022;53(5):1813–1822.
- Strain WD, et al. Effects of semaglutide on stroke subtypes in type 2 diabetes: post hoc analysis of the randomized SUSTAIN 6 and PIONEER 6. Stroke. 2022;53(9):2749–2757.
- Mariscal M, Testai FD. Emerging treatments for obesity: the role of GLP1 receptor agonists on stroke. Curr Neurol Neurosci Rep. 2025;25(1):36.
- Gera A, et al. Efficacy of glucagon-like peptide-1 receptor agonists for prevention of stroke among patients with and without diabetes: a meta-analysis with the SELECT and FLOW trails. Int J Cardiol Heart Vasc. 2025;57:101638. View this article via: PubMed Google Scholar
- Vergès B, et al. Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms. Cardiovasc Diabetol. 2022;21(1):242.
- Gunturu S. The potential role of GLP-1 agonists in psychiatric disorders: a paradigm shift in mental health treatment. Indian J Psychol Med. 2024;46(3):193–195.
- López-Ojeda W, Hurley RA. Glucagon-like peptide 1: an introduction and possible implications for neuropsychiatry. J Neuropsychiatry Clin Neurosci. 2024;36(2):A4–86.
- Zhang L, et al. Exploring glucagon-like peptide-1 receptor agonists as potential disease-modifying agent in psychiatric and neurodevelopmental conditions: evidence from a drug target Mendelian randomization. BMC Psychiatry. 2025;25(1):484.
- Pierret ACS, et al. Glucagon-like peptide 1 receptor agonists and mental health: a systematic review and meta-analysis. JAMA Psychiatry. 2025;82(7):643–653.
- Wen J, et al. Next generation dual GLP-1/GIP, GLP-1/glucagon, and triple GLP-1/GIP/glucagon agonists: a literature review. Nutr Metab Cardiovasc Dis. 2025;35(12):104213.
- Anastasiou IΑ, et al. Dual and triple gut peptide agonists on the horizon for the treatment of type 2 diabetes and obesity. An overview of preclinical and clinical data. Curr Obes Rep. 2025;14(1):34.
- Alfaris N, et al. GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review. EClinicalMedicine. 2024;75:102782.
- Cao L, et al. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson’s disease by reducing chronic inflammation in the brain. Neuroreport. 2016;27(6):384–391.
- Tai J, et al. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1678:64–74.
- Kopp KO, et al. Incretin-based multi-agonist peptides are neuroprotective and anti-inflammatory in cellular models of neurodegeneration. Biomolecules. 2024;14(7):872.
- Knerr PJ, et al. Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice. Mol Metab. 2022;63:101533.
- Müller TD, et al. Glucose-dependent insulinotropic polypeptide (GIP). Mol Metab. 2025;95:102118.
- Hölscher C. Glucagon-like peptide-1 class drugs show clear protective effects in Parkinson’s and Alzheimer’s disease clinical trials: A revolution in the making? Neuropharmacology. 2024;253:109952.
- Rhea EM, et al. Brain uptake pharmacokinetics of albiglutide, dulaglutide, tirzepatide, and DA5-CH in the search for new treatments of Alzheimer’s and Parkinson’s diseases. Tissue Barriers. 2024;12(4):2292461.
- Salameh TS, et al. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem Pharmacol. 2020;180:114187.
- Christensen M, et al. Transfer of liraglutide from blood to cerebrospinal fluid is minimal in patients with type 2 diabetes. Int J Obes (Lond). 2015;39(11):1651–1654.
- Hogg E, et al. A phase II, randomized, double-blinded, placebo-controlled trial of liraglutide in Parkinson’s disease [preprint]. https://doi.org/10.2139/ssrn.4212371 Posted on Lancet September 12, 2022.
- Wong CK, et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 2024;36(1):130–143.
- Kuroki T, et al. Exendin-4 inhibits matrix metalloproteinase-9 activation and reduces infarct growth after focal cerebral ischemia in hyperglycemic mice. Stroke. 2016;47(5):1328–1335.
- Xie Z, et al. Exendin-4 preserves blood-brain barrier integrity via glucagon-like peptide 1 receptor/activated protein kinase-dependent nuclear factor-kappa B/matrix metalloproteinase-9 inhibition after subarachnoid hemorrhage in rat. Front Mol Neurosci. 2021;14:750726.
- Rodriguez PJ, et al. Discontinuation and reinitiation of dual-labeled GLP-1 receptor agonists among US adults with overweight or obesity. JAMA Netw Open. 2025;8(1):e2457349.
- P S, et al. Factors associated with weight loss response to GLP-1 analogues for obesity treatment: a retrospective cohort analysis. BMJ Open. 2025;15(1):e089477.
- Papamargaritis D, et al. Effectiveness of integrating a pragmatic pathway for prescribing liraglutide 3.0 mg in weight management services (STRIVE study): a multicentre, open-label, parallel-group, randomized controlled trial. Lancet Reg Health Eur. 2024;39:100853.
- X Z, et al. Predicting responsiveness to GLP-1 pathway drugs using real-world data. BMC Endocr Disord. 2024;24(1):269.
- Kyriakidou A, et al. Clinical and genetic predictors of glycemic control and weight loss response to liraglutide in patients with type 2 diabetes. J Pers Med. 2022;12(3):424.
- Apostolopoulou M, Koufakis T. Predicting treatment response to GLP-1 receptor agonists: still tossing the coin or doing better? Expert Opin Pharmacother. 2025;26(10):1113–1115.
- Athauda D, et al. What effects might exenatide have on non-motor symptoms in Parkinson’s disease: a post hoc analysis. J Parkinsons Dis. 2018;8(2):247–258.
- Heisz JJ, Waddington EE. The principles of exercise prescription for brain health in aging. Exerc Sport Mov. 2024;2(1):1–5.View this article via: CrossRef Google Scholar
- Sujkowski A, et al. The protective role of exercise against age-related neurodegeneration. Ageing Res Rev. 2022;74:101543.
- Ben Ezzdine L, et al. Physical activity and neuroplasticity in neurodegenerative disorders: a comprehensive review of exercise interventions, cognitive training, and AI applications. Front Neurosci. 2025;19:1502417.
- Kong F, et al. Glucagon-like peptide 1 (GLP-1) receptor agonists in experimental Alzheimer’s disease models: a systematic review and meta-analysis of preclinical studies. Front Pharmacol. 2023;14:1205207.
- Teixeira LCR, et al. Exploring the role of GLP-1 receptor agonists in Alzheimer’s disease: a review of preclinical and clinical evidence. Receptors. 2025;4(1):2.
- Liang Y, et al. Clinical evidence for GLP-1 receptor agonists in Alzheimer’s disease: a systematic review. J Alzheimers Dis Rep. 2024;8(1):777–789.
- Kalinderi K, et al. GLP-1 receptor agonists: a new treatment in Parkinson’s disease. Int J Mol Sci. 2024;25(7):3812.
- Helal MM, et al. GLP-1 receptor agonists in Parkinson’s disease: an updated comprehensive systematic review with meta-analysis. Diabetol Metab Syndr. 2025;17(1):352.
- Dahiya S, et al. The effect of GLP-1 receptor agonists in pre-clinical rodent models of Parkinson’s disease: a systematic review and meta-analysis. Clin Park Relat Disord. 2022;6:100133. View this article via: PubMed Google Scholar
- Messak M, et al. Efficacy and safety of GLP-1 agonists in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(8):9721–9736.
- Sayed NH, et al. Vildagliptin attenuates Huntington’s disease through activation of GLP-1 receptor/PI3K/Akt/BDNF pathway in 3-nitropropionic acid rat model. Neurotherapeutics. 2020;17(1):252–268.
- Shirani A, Stuve O. Glucagon-like peptide-1 receptor agonists in multiple sclerosis: therapeutic promise, challenges, and future directions. Expert Opin Investig Drugs. 2025;34(11):929–941.
- Balshi A, et al. Glucagon-like peptide-1 agonist safety and efficacy in a multiple sclerosis cohort. Mult Scler Relat Disord. 2025;93:106229.
- Yang X, et al. Neuroprotective mechanisms of glucagon-like peptide-1-based therapies in ischemic stroke: an update based on preclinical research. Front Neurol. 2022;13:844697.
- Ali M, et al. Efficacy of GLP-1 agonists in psychiatric illnesses: a scoping review. Prim Care Companion CNS Disord. 2025;27(3):24nr03828. View this article via: PubMed Google Scholar
- Sa B, et al. Psychiatric effects of GLP-1 receptor agonists: a systematic review of emerging evidence. Diabetes Obes Metab. 2026;28(1):50–59.
-
Version history
- Version 1 (February 16, 2026): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Review Series
Clinical innovation and scientific progress in GLP-1 medicine
-
The science of safety: adverse effects of GLP-1 receptor agonists as glucose-lowering and obesity medications
Ryan J. Jalleh et al. -
GLP-1RA precision medicine in people with type 2 diabetes: current insights and future prospects
Pedro Cardoso et al. -
GLP-1 receptor agonists and cancer: current clinical evidence and translational opportunities for preclinical research
Estefania Valencia-Rincón et al. -
GLP-1 physiology and pharmacology along the gut-brain axis
Lisa R. Beutler -
The promise of GLP-1 receptor agonists for neurodegenerative diseases
Dilan Athauda et al. -
GLP-1 and the cardiovascular system
Florian Kahles et al. -
GLP-1 agonists in the treatment of chronic kidney disease in type 2 diabetes and obesity
Mark E. Cooper et al. -
Antiinflammatory actions of glucagon-like peptide-1–based therapies beyond metabolic benefits
Chi Kin Wong et al. -
Signaling architecture of the glucagon-like peptide-1 receptor
Gregory Austin et al.
Metrics



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)