Advertisement
Review Series Free access | 10.1172/JCI80009
Therapeutic cancer vaccines
Cornelis J.M. Melief,1,2 Thorbald van Hall,1 Ramon Arens,1 Ferry Ossendorp,1 and Sjoerd H. van der Burg1
1Leiden University Medical Center, Leiden, Netherlands.
2ISA Pharmaceuticals, Leiden, Netherlands.
Address correspondence to: Cornelis J.M. Melief, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC Leiden, Netherlands. Phone: 0031.71.5263800; E-mail: c.melief@lumc.nl.
Find articles by Melief, C. in: PubMed | Google Scholar
1Leiden University Medical Center, Leiden, Netherlands.
2ISA Pharmaceuticals, Leiden, Netherlands.
Address correspondence to: Cornelis J.M. Melief, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC Leiden, Netherlands. Phone: 0031.71.5263800; E-mail: c.melief@lumc.nl.
Find articles by van Hall, T. in: PubMed | Google Scholar
1Leiden University Medical Center, Leiden, Netherlands.
2ISA Pharmaceuticals, Leiden, Netherlands.
Address correspondence to: Cornelis J.M. Melief, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC Leiden, Netherlands. Phone: 0031.71.5263800; E-mail: c.melief@lumc.nl.
Find articles by Arens, R. in: PubMed | Google Scholar
1Leiden University Medical Center, Leiden, Netherlands.
2ISA Pharmaceuticals, Leiden, Netherlands.
Address correspondence to: Cornelis J.M. Melief, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC Leiden, Netherlands. Phone: 0031.71.5263800; E-mail: c.melief@lumc.nl.
Find articles by Ossendorp, F. in: PubMed | Google Scholar
1Leiden University Medical Center, Leiden, Netherlands.
2ISA Pharmaceuticals, Leiden, Netherlands.
Address correspondence to: Cornelis J.M. Melief, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC Leiden, Netherlands. Phone: 0031.71.5263800; E-mail: c.melief@lumc.nl.
Find articles by van der Burg, S. in: PubMed | Google Scholar
Published July 27, 2015 - More info
J Clin Invest. 2015;125(9):3401–3412. https://doi.org/10.1172/JCI80009.
Copyright © 2015, American Society for Clinical Investigation
-
Abstract
The clinical benefit of therapeutic cancer vaccines has been established. Whereas regression of lesions was shown for premalignant lesions caused by HPV, clinical benefit in cancer patients was mostly noted as prolonged survival. Suboptimal vaccine design and an immunosuppressive cancer microenvironment are the root causes of the lack of cancer eradication. Effective cancer vaccines deliver concentrated antigen to both HLA class I and II molecules of DCs, promoting both CD4 and CD8 T cell responses. Optimal vaccine platforms include DNA and RNA vaccines and synthetic long peptides. Antigens of choice include mutant sequences, selected cancer testis antigens, and viral antigens. Drugs or physical treatments can mitigate the immunosuppressive cancer microenvironment and include chemotherapeutics, radiation, indoleamine 2,3-dioxygenase (IDO) inhibitors, inhibitors of T cell checkpoints, agonists of selected TNF receptor family members, and inhibitors of undesirable cytokines. The specificity of therapeutic vaccination combined with such immunomodulation offers an attractive avenue for the development of future cancer therapies.
-
Introduction
Preventive vaccination against infectious diseases is considered one of the most successful health measures of all time. Therapeutic vaccination against established diseases such as persistent infections and cancer has proven much more challenging, because the vaccine intervention must combat an immune system that has been restrained by tolerizing or polarizing mechanisms that sustain the disease in a misguided attempt at self-tolerance. Nevertheless, recent clinical results indicate that the era of successful therapeutic vaccination has arrived. In this Review, we discuss the most attractive preclinical and clinical therapeutic vaccination strategies, as well as opportunities to improve such therapies. With the exception of some forms of premalignant disease, the proportion of patients benefiting from treatment with cancer vaccines, in addition to the mean survival advantages, leaves much to be desired. Better results can most likely be obtained by a better choice of antigens, improvements in vaccine design, and appropriate cotreatments. The latter can alleviate immunosuppressive mechanisms in the cancer microenvironment and boost vaccine performance by appropriate stimulation or modulation of the immune system.
-
Clinical cancer vaccines against nonviral antigens
Antigens on nonviral cancers are targeted for immunotherapy, including vaccines, for two main reasons: (a) the antigens can elicit an immune response that selectively attacks cancer cells, and (b) these antigens are (over-)expressed on cancer cells. If such antigens are expressed at all on normal cells, as in the case of differentiation antigens, the immune response to the normal tissues should only cause nonlethal side effects, such as vitiligo in the case of immune responses elicited against melanocyte antigens. A recent review has detailed clinical therapeutic vaccination studies in patients with nonviral cancers (1). In many phase I/II studies, these vaccines have shown clinical benefit, in particular extended overall or disease-free survival, while objective durable regressions of the type associated with targeted or immunomodulatory mAb therapy (2–6) or chimeric antigen receptor (CAR) (7–10) or adoptive T cell (11, 12) therapy were rarely seen.
Vaccines for nonviral cancers have targeted shared antigens. Vaccines against nonviral cancers have largely utilized target molecules, such as differentiation antigens, cancer testis (CT) antigens, or overexpressed antigens (1), that are common to a particular cancer type. A list of antigens commonly used in therapeutic vaccination against nonviral cancers is provided in Table 1 and refs. 1 and 13–24.
Central immunological tolerance mechanisms shape the T cell repertoire that recognizes these antigens (20, 25–27); thus, the T cells induced by these vaccines must rely on the T cell repertoire left after the induction of central tolerance, which depletes many, but not all, of the high-avidity T cells directed against such antigens. Indeed, overexpressed CT or differentiation antigens were found in medullary thymic epithelial cells that express virtually all self-molecules (25, 27), including cancer–associated antigens (28), although epitope expression failure can occur in the thymus (29). Nevertheless, deletional immunological tolerance of the T cell repertoire toward self-antigens is the rule rather than the exception.
Despite the likelihood of elimination through central tolerance mechanisms, adequate T cell repertoires are available to allow clinical benefit. Provenge (sipucleucel-T), which targets the prostate differentiation antigen prostate acid phosphatase (PAP), was the first cancer vaccine to be approved in the US and Europe on the basis of its capacity to prolong overall survival in patients with hormone-resistant prostate cancer by an average of 3 months (22). This vaccine is a cellular product generated from autologous peripheral blood monocytes (PBMCs) by culturing with a fusion protein of PAP linked to granulocyte-macrophage CSF (GM-CSF). The exact mode of action is not known, because the cultured PBMCs contain both partially activated DCs and T cells as well as other peripheral blood cellular components. At any rate, vaccination increased the number of PAP-specific T cells in the prostate (30). PROSTVAC-VF (TRICOM, Bavarian Nordic, exclusive option of BMS) (31, 32) is a cancer vaccine that consists of two recombinant viral vectors. Each vector encodes prostate-specific antigen (PSA) and three costimulatory molecules (CD80, ICAM-1, and LFA-3). Priming is achieved by a vaccinia virus vector, followed by a boost with fowlpox vector. Such a heterologous prime-boost protocol ensures that the response against the tumor-associated antigen (TAA), the only antigen shared between the two viral vectors, is enhanced by the boost. An increase in PSA-doubling time was observed that was associated with a survival benefit of 8.5 months in the vaccinated group versus a control group of patients with hormone-resistant prostate cancer (21).
Therapeutic vaccines have also been evaluated in patients with breast cancer, lung cancer, melanoma, pancreatic cancer, colorectal cancer, and renal cancer (1). A survival advantage was seen with some vaccines in phase II trials that was sometimes associated with an immune response to the vaccine in renal cell carcinoma (33), but no objective cancer regressions were noted. Survival advantage was also noted in a study of an HLA-A2–binding gp100-derived short peptide vaccine together with IL-2, versus treatment with IL-2 alone in patients with advanced melanoma (34). Target antigens for different cancers are listed in Table 1.
Mesothelin is a cell-surface molecule that is overexpressed on a variety of malignancies, including mesothelioma, pancreatic cancer, lung cancer, and ovarian cancer. Cancer regressions without appreciable toxicity were seen with an Ab-based immunotoxin against this target (reviewed in ref. 16). In one study of a Listeria-based vaccine incorporating mesothelin together with an allogeneic pancreatic cancer–based G-VAX vaccine in a prime-boost approach, a median survival of 6.1 months was noted in patients with advanced pancreatic cancer versus a median survival of 3.9 months for patients treated with the G-VAX vaccine alone (35). G-VAX vaccine consists of allogeneic cancer cell lines transduced with GM-CSF; in principle, vaccination with such cells protects against all immunogenic antigens in these lines that are shared with the patient’s cancer, with the exception of unique neoantigens.
Another vaccine approach in which antigen identification is bypassed involves the use of autologous tumor lysates (36, 37), which, in theory, have the advantage of incorporating the full range of neoantigens resulting from somatic mutations, without having to identify these neoantigens directly. The disadvantage is that one does not know how effective the processing of neoantigen epitopes from the lysate is for effective DC presentation. Also, the lysate, if presented in a highly immunogenic vaccine formulation, may conceivably induce autoimmunity to autoantigens also represented in the lysate.
In hematological malignancies, vaccination against the idiotype of monoclonal surface Ig on malignant B cells has been associated with prolonged disease-free survival in a phase III vaccine trial (38). However, regular anti–B cell mAbs, bispecific T cell–enhancing (BITE) Abs (39), and CARs targeting CD19 will likely supplant anti-idiotype approaches because of off-the-shelf availability or greater effectiveness.
In considering other vaccine approaches, in particular DC vaccines, it is noteworthy that Wilms tumor-1 (WT-1) peptide vaccination or vaccination with DCs electroporated with WT1 mRNA in patients with acute myelogenous leukemia (AML) has led to occasional leukemia regression (40, 41) or conversion from partial to complete remission following vaccination (42). Therapeutic vaccination with ex vivo–prepared tumor antigen–loaded DCs, particularly in patients with metastatic melanoma, has been practiced by numerous groups, mainly with monocyte-derived, in vitro–cultured DCs. Recently, a shift from such DCs to natural DC subsets occurring in blood and similarly loaded with TAAs has shown a tendency toward better clinical results (43). Also, whereas ex vivo–generated DCs were mostly loaded with class I MHC epitopes, CD4+ Th cell targeting has improved the clinical results (44). These results were confirmed by a study of in vitro interactions between DCs and CD4+ and CD8+ T cells (45). A skin test measuring infiltration by antigen-driven T lymphocytes reportedly predicts patient survival (46). DC vaccination with mRNA-encoding melanocyte-associated antigens electroporated into DCs following complete resection of metastases has shown encouraging survival results (47). However, apart from the results with sipuleucel-T, which hardly qualifies as a DC vaccine sensu stricto, no successful phase III trial results have been reported for DC-based cancer vaccines.
Emerging neoantigens for therapeutic vaccination against nonviral cancers. In the hierarchy of therapeutic vaccine targets in nonviral cancers, powerful arguments for selecting neoantigens (based on mutation) in addition, or even exclusively, whenever possible, were recently provided by both preclinical and clinical observations. Vaccines against mutation-based neoantigens are unique to each patient, because the mutations induced by carcinogens or UV light are random. Of course, the preparation of personalized vaccines poses new challenges.
The first evidence of the potential of neo-epitope vaccination was made in the B16 mouse melanoma model, in which synthetic long peptide (SLP) vaccination against two mutant antigens was associated with a marked antitumor effect (48, 49). More recently, in preclinical mouse experiments with a chemically induced escape variant tumor that had lost a major mutant rejection epitope, tumor rejection could still be achieved by Ab-mediated immune checkpoint blockade (anti–CTLA-4 or anti–PD-1). This effective treatment was demonstrated to act by reactivation of existing T cells against two other mutant CTL epitopes (50). Interestingly, the same therapeutic effect was also accomplished by vaccination with two SLPs incorporating these two mutant epitopes together with TLR3 ligand polyinosinic poylycitidylic acid (poly I:C) (50). Similar observations were made in an independent mutation–based cancer model (51). Likewise, in a clinical study of patients with metastatic melanoma, clinical benefit from treatment with anti–CTLA-4 was strongly associated with a high mutational load in the patients’ cancer, indicating that the responses to neoantigens that were unleashed by the anti–CTLA-4 treatment were clinically useful (52, 53). Anti–CTLA-4 treatment of patients with metastatic melanoma reportedly broadens the T cell responses to shared antigens such as overexpressed antigens, differentiation antigens, and CT antigens (54). The contribution to clinical success of this broadening, next to the arousal of T cell responses against neoantigens, is currently unknown, although evidence is emerging that adoptive T cell therapy with T cells directed against mutations is more effective than that directed against differentiation antigens. In any case, it has now been shown beyond doubt that tumor-infiltrating lymphocytes (TILs) from melanoma patients that successfully eradicate tumors frequently contain CD8+ or CD4+ T cells against neoantigens that are likely responsible for, or heavily contribute to, anticancer effects (53, 55–57)
Antigens of choice in virus-induced cancers. Cancers caused by viruses and other infectious agents such as Helicobacter pylori constitute approximately 20% of all cancers worldwide (58, 59). A list of the currently identified cancer viruses is shown in Table 2. Notably, preventative vaccines are available for only two of the human oncogenic virus types: hepatitis B virus (HBV) and HPV. Therapeutic vaccines against the viruses listed in Table 2 are attractive for the treatment of persistent viral infection or (pre-)malignant disease.
-
Clinical experience with therapeutic vaccines against human cancer viruses
HBV and hepatitis C virus vaccines. Despite the fact that preventive vaccines for HBV have been available for approximately 32 years, hundreds of millions of individuals worldwide are still persistently infected with HBV (60). Thus, therapeutic vaccines will be needed for years to come.
Several types of therapeutic vaccines against persistent HBV infection and its sequelae have been developed. These vaccine platforms include recombinant HBV proteins, DNA vaccines, recombinant virus vaccines, and subviral particles, as well as immune complexes of HBV surface antigen (HBsAg) and IgG anti-HBsAg (reviewed in ref. 61). Immune complex targeting through Fc receptors facilitates both DC ingestion of antigen and DC activation. Vaccination with these immune complexes consisting of HBsAg and IgG Abs yielded promising results in a phase II trial (62), but a phase III randomized trial showed no clinical or virological benefit in patients persistently infected with HBV (63). Therapeutic vaccines against hepatitis C virus (HCV) have utilized by and large the same vaccination platforms as those for vaccines against HBV (64). So far, good immunogenicity data have been collected for some vaccines, but no efficacy data are available as of this writing (64). HBV and HCV do not contain oncogenic proteins that need to remain expressed in the transformed cells, but rather cause hepatocellular cancer (HCC) by indirect mechanisms such as inflammatory events. This necessitates targeting persistent viral infection before malignant transformation, because HCC may not necessarily express viral proteins.
EBV vaccines. Immunotherapy for malignant disease caused by EBV is a classical success story for adoptive transfer of EBV-specific T cells in diseases such as post-transplantation EBV-induced lymphomas (11), but therapeutic vaccination against EBV-related diseases is still relatively underdeveloped. A report was published more than 10 years ago regarding a vaccine candidate consisting of a recombinant pox virus vector incorporating the EBV antigens EBNA-1 and LMP2 that elicited both CD4+ and CD8+ T cell responses against EBV. Recent results from two different phase I toxicity/immunogenicity trials testing this vaccine in patients with EBV-induced nasopharyngeal carcinoma (NPC) or other EBV-induced malignancies (65–67) showed that the vaccine was well tolerated and elicited antigen-specific T cell responses. An EBV vaccine could potentially prevent the development of EBV-induced malignancies, even if it does not prevent transmission of the virus (68). Considering the high proportion of individuals persistently infected with EBV (68), it is surprising how few groups actively work on the development of effective EBV vaccines.
Human T lymphotrophic virus-1 vaccines. Human T lymphotrophic virus-1 (HTLV-1) is a retrovirus that causes adult T cell leukemia/lymphoma (ATLL) or spastic paresis in a small proportion of persistently infected individuals. In a recent study, three patients with ATLL who were previously treated with conventional chemotherapy were subsequently vaccinated with DCs loaded with HLA-A2 epitopes of the viral tax protein, which is involved in both neoplastic transformation and spastic paresis induction. Two of these patients showed a partial response and the third later achieved a durable, complete response (69).
Merkel cell carcinoma virus vaccine. The Merkel cell carcinoma virus was only recently identified as the cause of a rare, rapidly metastasizing skin cancer (70, 71). It generates two virus-encoded oncogenic proteins: large T and small T. A patient with metastatic Merkel cell carcinoma could be successfully treated by adoptive transfer of T cells directed against the virus in combination with intratumoral injection of IFNβ-1b or low-dose lesion irradiation (72). In a preclinical therapeutic vaccination model, mice bearing small T–expressing B16 melanoma tumors lived significantly longer after DNA vaccination with a construct encoding small T than did animals that were vaccinated with empty vector. Small T vaccination was associated with substantial T cell response induction. This model is likely to predict clinical efficacy for this aggressive cancer type, because small T is as much a foreign antigen to patients as it is to mice (73).
HPV vaccines. Preventive HPV vaccines have been offered to 10- to 12-year-old girls for the past decade; however, compliance with vaccination is far from 100%, and the vaccines covering the high-risk types of HPV (HPV16 and -18, which are implicated in approximately 65% of all cervical cancers) have not yet been introduced in those parts of the world where they are needed the most. Even in the Western world, many individuals are persistently infected with high-risk HPV and are at risk of developing cervical cancer and HPV-positive head and neck cancer due to incomplete vaccine coverage and to the large cohort of infected individuals from the pre-HPV vaccine era (74–76).
The largest number of clinical studies using therapeutic vaccines in virally induced premalignant disease or cancer has been conducted to examine high-risk HPV, in particular HPV16, a virus responsible for approximately 50% of cervical carcinomas and 80% of HPV-positive head and neck cancers (reviewed in refs. 77–82). Thus far, the best immunogenicity in terms of CD4+ and CD8+ T cell responses and clinical responses was seen in patients with premalignant diseases such as cervical intraepithelial neoplasia (CIN) or vulvar intraepithelial neoplasia (VIN). Vaccination with SLPs that overlap the entire sequence of the E6 and E7 oncogenic proteins delivered s.c. in Montanide ISA-51 adjuvant induced robust CD4+ Th responses against many epitopes of these proteins and a somewhat less broad CD8+ T cell response (83, 84). More than 50% of patients had achieved partial or complete regression of lesions at three months after vaccination, and this percentage was even greater at twelve months after the last vaccine dose (83, 84). Moreover, there was a highly significant correlation between the strength of the vaccine-induced T cell immune response and the clinical response (83, 84). Interestingly, in patients with recurrent cervical cancer, this vaccine was much less immunogenic, and the induced T cell responses remained below the levels seen in VIN patients with clinical responses; indeed, vaccination did not confer a survival benefit of vaccination compared with historical controls (85). This illustrates one of the main problems in therapeutic cancer vaccination: cancer-associated alteration of systemic and local immunity appears to have a deleterious effect on T cell immunocompetence. In the treatment of VIN, results similar to those with SLP vaccination were achieved by application of Imiquimod ointment on the lesions, followed by vaccination with TA-CIN, a fusion protein of HPV16 E6 and E7 and the viral capsid protein L2 (86). DNA vaccines delivered by electroporation also induced a robust T cell response (87, 88) and lesion regression, together with virus clearance (88). Another promising vaccine is based on recombinant Listeria-E7 bacteria (89).
CMV vaccines. In recent years, CMV expression was reported in a high proportion of patients with glioblastomas. Although no causal role of CMV in the genesis of these tumors has been established, an oncomodulatory role is possible, and CMV antigens in these tumors can serve as targets for cancer vaccines (90, 91).
Common themes in therapeutic vaccines against human cancer viruses. Like the vaccines directed against neoantigens, the therapeutic vaccines against cancer viruses are directed against viral proteins that are not subject to central thymic tolerance. Moreover, many of these vaccines are directed against viral structures involved in malignant transformation (Table 2). This advantage can be exploited, because the vaccine can deliver antigen and stimulate robust effector T cell responses, whereas the natural infection is subject to the myriad intricate immune evasion and immunosuppression mechanisms these viruses have developed in the course of evolution (92–98). Nevertheless, these vaccines have to work against the same peripheral cell tolerance–, anergy–, and suppression–driving mechanisms operating in nononcogenic, persistent viral infections and nonviral cancers. These mechanisms include checkpoint suppression of T cells (4–6, 50, 99–101), Tregs (102), myeloid-derived suppressor cells (MDSCs) (103, 104), immunosuppressive cytokines such as TGF-β (105, 106) and IL-10 (107–109), and indoleamine 2,3-dioxygenase (IDO) (110–112), as well as lack of T cell infiltration at cancer sites (113–116) and improper inflammation (e.g., IL-6 mediated) (117–119).
-
Guidelines for the development of successful therapeutic cancer vaccines
Therapeutic cancer vaccines must achieve sufficient antigen concentration in DCs. Therapeutic vaccines need a rational vaccine design that achieves concentrated antigen delivery to DCs and DC activation, which in turn drives both CD4+ and CD8+ T cell responses (Figures 1 and 2). CD4+ T cells are needed for optimal and sustained effector CD8+ T cell responses (120–124) as well as induction and maintenance of CD8+ memory (125, 126). Quite apart from these CD8+ T cell–supporting roles, CD4+ T cells have intrinsic effector functions (121, 127). Despite these insights, many therapeutic cancer vaccines have consisted of short, exact HLA class I–binding peptides (usually HLA-A2 binding), which do not induce CD4+ T cells, resulting in short-lived CD8+ T cell responses.
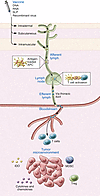 Figure 1
Figure 1Mode of action of therapeutic cancer vaccines. Routes of vaccine administration and migration of immune cells. Antigen-loaded DCs (APCs) travel through the afferent lymph to the lymph nodes, where they prime T cells. The primed, activated T cells migrate through the efferent lymph, thoracic duct, and blood to reach tumor cells. Vaccine-induced T cells must engage with and overcome hostile elements in the cancer microenvironment, including immunosuppressive cells (Tregs, MDSCs) and factors released by the tumor cells, such as immunosuppressive chemokines and cytokines and IDO, which impair T cell migration, function, and expansion.
Short peptides (<15 amino acids) do not require processing by professional antigen-presenting cells (APCs) and therefore bind exogenously to the HLA class I molecules of all nucleated cells that have surface HLA class I. Thus, most of these injected short peptides will end up in nonphysiologically large numbers, clogging the appropriate HLA class I molecules of nonprofessional APCs in the absence of costimulatory molecules. This is basically a tolerizing signal, as we have previously shown (128–130). In the case of short peptide vaccination in incomplete Freund’s adjuvant (IFA), the T cells elicited by vaccination traveled to the vaccination site instead of to the tumor and appeared to die there (131). In the same study (131), vaccination with SLPs did not lead to such tolerance and was associated with proper antitumor activity, completely in line with our findings with short and long peptides in IFA (128). Indeed, effector CD8+ T cell induction by short peptides and the associated antitumor effect is much less efficient than what is observed following vaccination with long peptides encompassing the same CD8+ T cell epitope. Also, vaccination with long peptides in IFA or its close relative Montanide ISA-51 supports robust T cell responses to SLPs, but not to most short peptides (128, 131–135). Only ex vivo loading of preactivated DCs with short peptides (136) or replacement of the CD4+ helper signal with agonist anti-CD40 Ab, with or without TLR ligand poly I:C (137, 138) or with CpG (139), can circumvent such tolerance induction against short, MHC class I–binding peptides in mice or patients.
SLPs (>20 amino acids) are pro-drugs in the sense that they are not biologically active by themselves, but need additional processing to allow loading in DC HLA molecules. The antigen presentation resulting from SLP vaccination reflects physiological pathways associated with much lower, and therefore more appropriate, MHC-ligand presentation than the uncontrollable and usually too-high peptide loading resulting from short peptide vaccination (Figure 2). We and others have shown that only DCs are capable of efficiently processing such SLPs for presentation in both MHC class I and class II molecules (140, 141) and that such processing is much more efficient than that of intact proteins (141, 142). Moreover, SLPs typically harbor both CD4+ and CD8+ T cell epitopes, ensuring that vaccination with SLPs induces a balanced CD4/CD8 response.
 Figure 2
Figure 2Processing of vaccine-derived antigens. For antigen loading in HLA class I molecules, antigen must enter the DC cytoplasm to be processed by the proteasome complex. Longer antigen fragments are cut down by the proteasome to smaller, 9– to 15–amino acid stretches that can be pumped through the transporter of antigen processing (TAP), thereby gaining access to the endoplasmic reticulum, where HLA class I loading with fragments of 9 to 12 amino acids takes place, followed by transport to the cell surface. For antigen loading in HLA class II molecules, the antigen must enter the endosomal system, where cathepsins digest the antigen, followed by loading of HLA class II with fragments of approximately 12 to 15 amino acids at low pH and, following DC maturation, transport to the cell surface. Once DCs have fully matured, they interact with CD8+ and CD4+ T cells by stimulating the TCR with antigen presented by HLA class I or II molecules, respectively, and costimulatory molecules such as CD28. Activated, primed CD8+ T cells are then capable of killing tumor cells via ligation of the TCR with antigen presented by HLA class I molecules.
Cancer vaccines must utilize an effective route of administration. The preferred routes of cancer vaccine administration must effectively target the antigen to DCs (Figures 1 and 2). This is best achieved by s.c. administration or by delivery into DC-rich lymph nodes (reviewed in ref. 143). Other effective routes include s.c. long peptide delivery in Montanide (83). DNA vaccines have also been delivered effectively by i.m. injection in combination with electroporation (87, 88). An important consideration is the extent to which adjuvants and vaccination routes contribute to the proper circulating and tissue-resident CD4+ and CD8+ T cells with a homing preference for the proper cancer-infiltrated tissues. Considerable insights into the different T cell subsets and specific tissue-homing patterns have been obtained from mouse infection models (reviewed in ref. 144). Recent data show that mucosal cancers are best treated by vaccines that endow T cells with mucosal homing properties (145), but much more work is needed before solid rules for more efficient homing to cancerous tissues are incorporated into human cancer vaccine designs.
Therapeutic cancer vaccines must activate DCs with adjuvants. A crucial requirement of the proper action of SLP vaccines is the inclusion of appropriate adjuvants, including TLR ligands such as poly I:CLC (TLR3 ligand), CpG (TLR9 ligand) (50, 132–135, 146), Montanide (77–81, 83, 84, 132–135), or stimulator of IFN genes (STING) agonists (147). SLP vaccines can be further improved by the covalent coupling of a powerful TLR ligand to the peptide, leading to superior DC targeting and simultaneous DC activation (148–150). DNA or RNA vaccines contain more or less powerful built-in DC activators such as TLR ligands and pattern recognition receptors (PRRs) (reviewed in refs. 151, 152). Other popular cancer vaccines, including PROSTVAC, have utilized viral vectors; however, while such vectors contain numerous PRR ligands capable of DC activation (152), they also contain sequences that compete with the inserted TAAs (78, 153). Although PROSTVAC extended patient survival (21), the performance of vaccines incorporating this prostate antigen may conceivably be improved by excluding potentially competing vector sequences and incorporating strong immunostimulants. Heterologous prime boosting overcomes this problem to some extent (21).
Cancer vaccines based on recombinant protein delivery have also been used extensively, such as in the MAGE-A3 recombinant protein vaccination trials, in patients with metastatic melanoma or metastatic lung cancer. Recombinant protein vaccines suffer from the serious disadvantage that processing of such proteins by DCs for presentation by HLA class I molecules to CD8 T cells is very inefficient, as illustrated in the case of the MAGE-A3 vaccine by the lack of a demonstrable CD8 T cell response (154, 155). Moreover, the primary endpoint (extension of disease-free survival) was not met in phase III MAGE-A3 protein vaccination trials (156–158).
In view of the overwhelming importance of activated DCs in the initiation of therapeutic CD4+ and CD8+ T cell responses (Figures 1 and 2), an attractive strategy consists of isolating these cells, loading them with antigen, activating and maturing them appropriately, and then administering them as a therapeutic vaccine to cancer patients. Although Provenge has been advertised as a DC vaccine, it is a rather complex cellular product whose mode of action has not been well defined. Other DC vaccines have been very well defined and in several instances have shown promising therapeutic results, mainly in patients with metastatic melanoma (43–47, 159). Although DC vaccines may become a successful treatment modality, the manufacture of such personalized vaccines is expensive and laborious and requires a sophisticated good manufacturing practices (GMP) setting. Thus, we argue that such vaccines can serve as proof-of-concept and mode-of-action studies, but they eventually need to be replaced by antigen delivery and DC activation systems, such as SLP-TLR ligand conjugates, that directly target the DCs in vivo (148, 149). In summary, with noted exceptions, the design of many cancer vaccines has insufficiently heeded the rules for efficient antigen processing and presentation in DCs for proper CD4+ and CD8+ effector and memory T cell induction and, by that criterion alone, have fallen short of one of the most important conditions for success.
Therapeutic cancer vaccines cannot be expected to act as a monotherapy. Cancer vaccines have been vilified because they do not approach the effectiveness of adoptive T cell transfer (160). However, the antigen specificity of adoptively transferred T cells (frequently expanded TILs) appears to determine the therapeutic efficacy of TILs. Recent evidence indicates that in a substantial number of cases, TILs are likely to have been directed against neoantigens (refs. 53, 57 and E.M. Verdegaal and S.H. van der Burg, personal communication), rather than against the shared antigens utilized as targets for most therapeutic vaccines against nonviral cancers. Indeed, persistence of CTL clones targeting melanocyte differentiation antigens was insufficient to mediate significant melanoma regression in patients (161). On the other hand, in metastatic melanoma patients, immunotherapy with adoptive transfer of T cells transduced with an avidity-enhanced, NY-ESO-1–specific T cell receptor (TCR) produced marked, durable tumor regression (12), indicating that CT antigens such as NY-ESO-1 may be better targets for immunotherapy than differentiation antigens. Personalized vaccines against immunodominant CD4+ Th and CD8+ CTL epitopes representing neoantigens may indeed have a greater chance of therapeutic success, as was recently shown with SLP vaccines in mouse models (50, 51, 162). Development of personalized vaccines will create novel logistical and regulatory challenges that will likely be overcome by new technologies for rapid epitope prediction and validation from cancer exome sequences (50, 51).
Effective adoptive T cell transfer therapy requires drastic lymphopenia, implemented by heavy pretreatment with chemotherapeutics and/or total body x-ray irradiation or IFN-α conditioning (163, 164). It is unreasonable to demand that cancer vaccines be effective without a similarly profound restructuring of the cancer microenvironment (Figure 3). Thus, additional treatment is a necessity, not only to allow the vaccines to induce proper effector and memory T cell populations in the systemic circulation, but also to allow these cells to migrate to the cancer sites and exert tumoricidal activity. Lymphopenia is obviously not the answer; however, many standard chemotherapeutic agents such as thalidomide derivatives and other targeted compounds are known to deplete immunosuppressive Tregs and/or MDSCs without affecting effector T cell and memory T cell populations (Figure 3B). Not surprisingly, such depletion sets the stage for improved performance of immunotherapies, including simultaneous therapeutic vaccination (reviewed in ref. 165). An additional mechanism of improved tumor eradication by chemoimmunotherapy is cisplatin-mediated lowering of the tumor apoptosis threshold by TNF-α released from vaccine-induced intratumoral T cells (166). Another obvious choice for combination treatment is the addition of checkpoint-blocking agents to therapeutic vaccination, which has yielded promising results in preclinical models (Figure 3B and refs. 167–169). The combination of therapeutic vaccines with mAbs targeting selected TNF receptor family members (CD40, refs. 138, 170, 171; 4-1 BB/CD137, refs. 172–174; OX-40/CD134, refs. 172, 174–176; and CD27, refs. 177, 178) or immunosuppressive cytokines (IL-10, refs. 179, 180; TGF-β, refs. 181–183; IL-6, refs. 184, 185) is also an attractive approach (Figure 3B). Finally, the combination of a cancer vaccine with γC cytokines such as IL-7, IL-15, and IL-21 or IL-2 (186) may also be effective, because each of these cytokines has the capacity to expand antigen-experienced T cells.
 Figure 3
Figure 3Methods to overcome the hostility of the cancer microenvironment toward T cells. (A) T cell–suppressive mechanisms include the production of immunosuppressive cytokines (IL-10, TGF-β), inflammatory cytokines (IL-6), IDO, and NO, and recruitment of immunosuppressive macrophages (M2-type tumor-associated macrophages [TAMs]) and MDSCs. Cancer cells do not provide the necessary “danger” signals for DC activation, permitting T cell effector and memory cell induction. Thus, DCs are not properly polarized to induce such responses, leading to Treg induction, T cell anergy, and T cell deletion. Moreover, inhibitory checkpoint control molecules such as CTLA-4, PD-1, TIM3, or LAG3 are upregulated on chronically and improperly stimulated T cells. (B) T cell–immunosuppressive mechanisms are counteracted by Abs against immunosuppressive and inflammatory cytokines or their cognate receptors, T cell–stimulatory Abs against TNF receptor family members (CD27, CD40, CD134, and CD137), chemotherapeutics causing immunogenic cell death, or IDO inhibitors. Importantly, vaccination must induce proper effector CD4+ and CD8+ T cell generation in lymph nodes. The robust circulating effector T cells induced by these vaccines travel to tumor sites, where their activity can be optimized by appropriate combinatorial therapies.
Epilogue. Despite designs that are frequently suboptimal, cancer vaccines have shown clinical activity, particularly in the increase of recurrence-free or overall survival. Obvious choices for the design of powerful cancer vaccines are those that offer the ability to induce robust effector CD4+ and CD8+ T effector and memory responses. Cancer vaccines with such high-performance capacity are RNA, DNA, and SLP vaccines with the appropriate added or built-in adjuvants. The choice of target antigens for incorporation into cancer vaccines is of crucial importance, and viral- and mutation-based neoantigens, which are not subject to central thymus-driven tolerance, constitute very attractive targets. Additionally, selected CT and overexpressed antigens have demonstrated induction of potent tumoricidal T lymphocytes, but the T cell repertoires available against these antigens must be carefully scrutinized. In principle, differentiation antigens are less attractive targets, because the T cell repertoire against these is usually blunted and because powerful effector T cell generation against these antigens may cause serious toxicity. Because of the many T cell–suppressive activities in the cancer microenvironment (Figure 3A), cancer vaccines cannot be expected to show optimal anticancer efficacy by themselves, but need to be used in combination treatments that are designed to inactivate the most important immunosuppressive mechanisms in this environment. Many precision drugs that block immunosuppressive activities were recently identified (Figure 3B), contributing to many exciting new possibilities within the vaccine branch of cancer immunotherapy. Therapeutic vaccines offer the prospect of highly specific cancer therapies that are relatively inexpensive and noninvasive and can be effective in combination with standard chemo- or radiation therapies or with immunomodulatory drugs.
-
Acknowledgments
Writing of this review was supported by Dutch Cancer Society grant KWO 2009-4400, project leaders C.J.M. Melief and S.H. van der Burg.
Address correspondence to: Cornelis J.M. Melief, Leiden University Medical Center, PO Box 9600, Leiden, 2300 RC Leiden, Netherlands. Phone: 0031.71.5263800; E-mail: c.melief@lumc.nl.
-
Footnotes
Conflict of interest: Cornelis J.M. Melief is fully employed as Chief Scientific Officer of ISA Pharmaceuticals and has stock appreciation rights in the company. Cornelis Melief and Sjoerd H. van der Burg are co-inventors on numerous patents and patent applications in the area of synthetic long peptide vaccines. Clinical trials conducted by Melief and van der Burg with synthetic long peptides have been funded by the Dutch Cancer Society and by ISA Pharmaceuticals.
Reference information: J Clin Invest. 2015;125(9):3401–3412. doi:10.1172/JCI80009.
-
References
- Melero I, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–524.
- Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465.
- Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715.
- Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723.
- Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454.
- Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133.
- Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828.
- Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. View this article via: PubMed Google Scholar
- Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517.
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733.
- Heslop HE, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935.
- Robbins PF, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2014;21(5):1019–1027.View this article via: PubMed Google Scholar
- Gameiro SR, Jammeh ML, Hodge JW. Cancer vaccines targeting carcinoembryonic antigen: state-of-the-art and future promise. Expert Rev Vaccines. 2013;12(6):617–629.
- He Y, Hong Y, Mizejewski GJ. Engineering α-fetoprotein-based gene vaccines to prevent and treat hepatocellular carcinoma: review and future prospects. Immunotherapy. 2014;6(6):725–736.
- Ohue Y, et al. Spontaneous antibody, and CD4 and CD8 T-cell responses against XAGE-1b (GAGED2a) in non-small cell lung cancer patients. Int J Cancer. 2012;131(5):E649–E658.
- Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74(11):2907–2912.
- Kessler JH, et al. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med. 2001;193(1):73–88.
- De Pas T, et al. Vaccines in non-small cell lung cancer: rationale, combination strategies and update on clinical trials. Crit Rev Oncol Hematol. 2012;83(3):432–443.
- Church SE, Jensen SM, Antony PA, Restifo NP, Fox BA. Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells. Eur J Immunol. 2014;44(1):69–79.
- Pedersen SR, Sorensen MR, Buus S, Christensen JP, Thomsen AR. Comparison of vaccine-induced effector CD8 T cell responses directed against self- and non-self-tumor antigens: implications for cancer immunotherapy. J Immunol. 2013;191(7):3955–3967.
- Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105.
- Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422.
- Xi HB, Wang GX, Fu B, Liu WP, Li Y. Survivin and PSMA loaded dendritic cell vaccine for the treatment of prostate cancer. Biol Pharm Bull. 2015;38(6):827–835.
- Martorelli D, et al. IGKV3 proteins as candidate “off-the-shelf” vaccines for κ-light chain-restricted B-cell non-Hodgkin lymphomas. Clin Cancer Res. 2012;18(15):4080–4091.
- Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140(1):123–135.
- Engelhard VH, Bullock TN, Colella TA, Sheasley SL, Mullins DW. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol Rev. 2002;188:136–146.
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9(12):833–844.
- Bos R, et al. Expression of a natural tumor antigen by thymic epithelial cells impairs the tumor-protective CD4+ T-cell repertoire. Cancer Res. 2005;65(14):6443–6449.
- Pinto S, et al. Misinitiation of intrathymic MART-1 transcription and biased TCR usage explain the high frequency of MART-1-specific T cells. Eur J Immunol. 2014;44(9):2811–2821.
- Fong L, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11):dju268.
- DiPaola RS, et al. A National Multicenter Phase 2 Study of Prostate-specific Antigen (PSA) Pox Virus Vaccine with Sequential Androgen Ablation Therapy in Patients with PSA Progression: ECOG 9802. Eur Urol. 2014;S0302-2838(14):01265–2.View this article via: PubMed Google Scholar
- Gulley JL, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2(2):133–141.
- Walter S, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261.
- Schwartzentruber DJ, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127.
- Le DT, et al. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes-Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33(12):1325–1333.
- Reardon DA, et al. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines. 2013;12(6):597–615.
- Joshi VB, Geary SM, Gross BP, Wongrakpanich A, Norian LA, Salem AK. Tumor lysate-loaded biodegradable microparticles as cancer vaccines. Expert Rev Vaccines. 2014;13(1):9–15.
- Thomas SK, Kwak LW. Lymphoma vaccine therapy: next steps after a positive, controlled phase III clinical trial. Semin Oncol. 2012;39(3):253–262.
- Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17(3):385–392.
- Ochsenreither S, et al. “Wilms Tumor Protein 1” (WT1) peptide vaccination-induced complete remission in a patient with acute myeloid leukemia is accompanied by the emergence of a predominant T-cell clone both in blood and bone marrow. J Immunother. 2011;34(1):85–91.View this article via: CrossRef Google Scholar
- Ochsenreither S, et al. Wilms’ tumor protein 1 (WT1) peptide vaccination in AML patients: predominant TCR CDR3beta sequence associated with remission in one patient is detectable in other vaccinated patients. Cancer Immunol Immunother. 2012;61(3):313–322.
- Van Tendeloo VF, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A. 2010;107(31):13824–13829.
- Wimmers F, Schreibelt G, Skold AE, Figdor CG, de Vries IJ. Paradigm shift in dendritic cell-based immunotherapy: from in vitro generated monocyte-derived DCs to naturally circulating DC subsets. Front Immunol. 2014;5:165.View this article via: PubMed Google Scholar
- Aarntzen EH, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73(1):19–29.
- Hoyer S, et al. Concurrent interaction of DCs with CD4(+) and CD8(+) T cells improves secondary CTL expansion: It takes three to tango. Eur J Immunol. 2014;44(12):3543–3559.
- Aarntzen EH, et al. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res. 2012;72(23):6102–6110.
- Wilgenhof S, et al. Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol Immunother. 2015;64(3):381–388.
- Kreiter S, Castle JC, Tureci O, Sahin U. Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology. 2012;1(5):768–769.
- Castle JC, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72(5):1081–1091.
- Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581.
- Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576.
- Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199.
- van Rooij N, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31(32):e439–e442.
- Kvistborg P, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6(254):254ra128.
- Tran E, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645.
- Linnemann C, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81–85.View this article via: PubMed Google Scholar
- Lu YC, et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res. 2014;20(13):3401–3410.
- de Martel C, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615.
- Vandeven N, Nghiem P. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol Res. 2014;2(1):9–14.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108.
- Kapoor R, Kottilil S. Strategies to eliminate HBV infection. Future Virol. 2014;9(6):565–585.
- Xu DZ, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS One. 2008;3(7):e2565.
- Xu DZ, et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol. 2013;59(3):450–456.
- Xue J, Zhu H, Chen Z. Therapeutic vaccines against hepatitis C virus. Infect Genet Evol. 2014;22:120–129.
- Taylor GS, et al. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J Virol. 2004;78(2):768–778.
- Hui EP, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013;73(6):1676–1688.
- Taylor GS, et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res. 2014;20(19):5009–5022.
- Cohen JI, Mocarski ES, Raab-Traub N, Corey L, Nabel GJ. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine. 2013;31(suppl 2):B194–B196.View this article via: PubMed Google Scholar
- Suehiro Y, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol. 2015;169(3):356–367.
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100.
- Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118–130.
- Chapuis AG, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol Res. 2014;2(1):27–36.
- Gomez B, He L, Tsai YC, Wu TC, Viscidi RP, Hung CF. Creation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigen. Cell Biosci. 2013;3(1):29.
- Bosch FX, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(suppl 8):I1–I31.View this article via: PubMed Google Scholar
- Hashibe M, Sturgis EM. Epidemiology of oral-cavity and oropharyngeal carcinomas: controlling a tobacco epidemic while a human papillomavirus epidemic emerges. Otolaryngol Clin North Am. 2013;46(4):507–520.
- Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82.
- Arens R, van Hall T, van der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Semin Immunol. 2013;25(2):182–190.
- Quakkelaar ED, Melief CJ. Experience with synthetic vaccines for cancer and persistent virus infections in nonhuman primates and patients. Adv Immunol. 2012;114:77–106.
- van Hall T, van der Burg SH. Mechanisms of peptide vaccination in mouse models: tolerance, immunity, and hyperreactivity. Adv Immunol. 2012;114:51–76.
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–360.
- van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol. 2011;23(2):252–257.
- Vici P, et al. Immunologic treatments for precancerous lesions and uterine cervical cancer. J Exp Clin Cancer Res. 2014;33(1):29.
- Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847.
- Welters MJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107(26):11895–11899.
- van Poelgeest MI, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88.View this article via: PubMed Google Scholar
- Daayana S, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102(7):1129–1136.
- Bagarazzi ML, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4(155):155ra138. View this article via: PubMed Google Scholar
- Kim TJ, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317.View this article via: PubMed Google Scholar
- Cory L, Chu C. ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccin Immunother. 2014;10(11):3190–3195.
- Landi D, Hegde M, Ahmed N. Human cytomegalovirus antigens in malignant gliomas as targets for adoptive cellular therapy. Front Oncol. 2014;4:338.View this article via: PubMed Google Scholar
- Mitchell DA, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369.
- Dustin LB, Cashman SB, Laidlaw SM. Immune control and failure in HCV infection — tipping the balance. J Leukoc Biol. 2014;96(4):535–548.
- Grabowska AK, Riemer AB. The invisible enemy — how human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol J. 2012;6:249–256.
- Hu Z, Usherwood EJ. Immune escape of γ-herpesviruses from adaptive immunity. Rev Med Virol. 2014;24(6):365–378.
- Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci. 2011;102(1):44–50.
- Karim R, et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte’s innate immune response. PLoS Pathog. 2013;9(5):e1003384.
- Liu S, et al. Human hepatitis B virus S and E antigens inhibit major vault protein signaling in interferon induction pathways. J Hepatol. 2015;62(5):1015–1023.
- Liu Y, et al. Hepatitis B virus polymerase disrupts K63-linked ubiquitination of STING to block innate cytosolic DNA-sensing pathways. J Virol. 2015;89(4):2287–2300.
- Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202.View this article via: CrossRef Google Scholar
- Sutmuller RP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832.
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366.
- Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7.
- Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110.
- Damuzzo V, et al. Complexity and challenges in defining myeloid-derived suppressor cells. [published online ahead of print November 26, 2014]. Cytometry B Clin Cytom. View this article via: CrossRef Google Scholar
- Mao Y, Poschke I, Kiessling R. Tumour-induced immune suppression: role of inflammatory mediators released by myelomonocytic cells. J Intern Med. 2014;276(2):154–170.
- Oh SA, Li MO. TGF-β: guardian of T cell function. J Immunol. 2013;191(8):3973–3979.
- Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol. 2013;25(6):637–645.
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228.
- Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–27.
- Lemos H, Huang L, McGaha TL, Mellor AL. Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur J Immunol. 2014;44(10):2847–2853.
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143.
- Platten M, von Knebel DN, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2014;5:673.View this article via: PubMed Google Scholar
- Chatterjee S, Behnam AB, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31–82.View this article via: PubMed Google Scholar
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022.
- Gorbachev AV, Fairchild RL. Regulation of chemokine expression in the tumor microenvironment. Crit Rev Immunol. 2014;34(2):103–120.
- Zumwalt TJ, Arnold M, Goel A, Boland CR. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget. 2015;6(5):2981–2991.View this article via: PubMed Google Scholar
- Mauer J, Denson JL, Bruning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36(2):92–101.
- Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54–74.
- Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746.
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480.
- Kast WM, Bronkhorst AM, de Waal LP, Melief CJ. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986;164(3):723–738.View this article via: PubMed Google Scholar
- Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187(5):693–702.
- Ridge JP, Di RF, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478.
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483.
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421(6925):852–856.
- Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93.
- Quezada SA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650.
- Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179(8):5033–5040.
- Toes RE, Offringa R, Blom RJ, Melief CJ, Kast WM. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci U S A. 1996;93(15):7855–7860.
- Toes RE, Blom RJ, Offringa R, Kast WM, Melief CJ. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J Immunol. 1996;156(10):3911–3918.View this article via: PubMed Google Scholar
- Hailemichael Y, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472.
- Zwaveling S, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169(1):350–358.
- Sabbatini P, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18(23):6497–6508.
- Tsuji T, et al. Effect of Montanide and poly-ICLC adjuvant on human self/tumor antigen-specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol Res. 2013;1(5):340–350.
- van Duikeren S, et al. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol. 2012;189(7):3397–3403.
- Toes RE, et al. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol. 1998;160(9):4449–4456.View this article via: PubMed Google Scholar
- Cho HI, Barrios K, Lee YR, Linowski AK, Celis E. BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 2013;62(4):787–799.
- Diehl L, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5(7):774–779.
- Speiser DE, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746.
- Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38(4):1033–1042.
- Rosalia RA, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. 2013;43(10):2554–2565.
- Zhang H, et al. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J Biol Chem. 2009;284(14):9184–9191.
- Silva JM, Videira M, Gaspar R, Preat V, Florindo HF. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J Control Release. 2013;168(2):179–199.
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161.
- Schijns V, Tartour E, Michalek J, Stathopoulos A, Dobrovolskiene NT, Strioga MM. Immune adjuvants as critical guides directing immunity triggered by therapeutic cancer vaccines. Cytotherapy. 2014;16(4):427–439.
- Welters MJ, et al. Multiple CD4 and CD8 T-cell activation parameters predict vaccine efficacy in vivo mediated by individual DC-activating agonists. Vaccine. 2007;25(8):1379–1389.
- Dubensky TW, Dubensky TW Jr, Kanne DB, Leong ML. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther Adv Vaccines. 2013;1(4):131–143.
- Khan S, et al. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem. 2007;282(29):21145–21159.
- Zom GG, et al. Efficient induction of antitumor immunity by synthetic toll-like receptor ligand-peptide conjugates. Cancer Immunol Res. 2014;2(8):756–764.
- Zom GG, Khan S, Filippov DV, Ossendorp F. TLR ligand-peptide conjugate vaccines: toward clinical application. Adv Immunol. 2012;114:177–201.
- Sahin U, Kariko K, Tureci O. mRNA-based therapeutics — developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780.
- van den Boorn JG, Barchet W, Hartmann G. Nucleic acid adjuvants: toward an educated vaccine. Adv Immunol. 2012;114:1–32.
- Bos R, et al. Characterization of antigen-specific immune responses induced by canarypox virus vaccines. J Immunol. 2007;179(9):6115–6122.
- Kruit WH, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31(19):2413–2420.
- Vansteenkiste J, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;31(19):2396–2403.
- GlaxoSmithKline. The investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoint in Phase III melanoma clinical trial [press release]. London, United Kingdom: September 5, 2013. http://www.gsk.com/en-gb/media/press-releases/2013/the-investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoint-in-phase-iii-melanoma-clinical-trial/. Accessed June 10, 2015.
- GlaxoSmithKline. Investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoints in MAGRIT, a phase III non-small cell lung cancer clinical trial [press release]. London, United Kingdom: March 20, 2014. http://www.gsk.com/en-gb/media/press-releases/2014/investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoints-in-magrit-a-phase-iii-non-small-cell-lung-cancer-clinical-trial/. Accessed June 10, 2015.
- GlaxoSmithKline. Update on phase III clinical trial of investigational MAGE-A3 antigen-specific cancer immunotherapeutic in non-small cell lung cancer [press release]. London, United Kingdom: April 2, 2014. http://www.gsk.com/en-gb/media/press-releases/2014/update-on-phase-iii-clinical-trial-of-investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-in-non-small-cell-lung-cancer/. Accessed June 10, 2015.
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10(5):475–480.
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915.
- Chandran SS, et al. Persistence of CTL clones targeting melanocyte differentiation antigens was insufficient to mediate significant melanoma regression in humans. Clin Cancer Res. 2015;21(3):534–543.
- Kreiter S, Diken M, Selmi A, Tureci O, Sahin U. Tumor vaccination using messenger RNA: prospects of a future therapy. Curr Opin Immunol. 2011;23(3):399–406.
- Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257(1):56–71.
- Verdegaal EM, et al. Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-α. Cancer Immunol Immunother. 2011;60(7):953–963.
- Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74(10):2663–2668.
- van der Sluis TC, et al. Vaccine-induced tumor necrosis factor-producing T cells synergize with Cisplatin to promote tumor cell death. Clin Cancer Res. 2015;21(4):781–794.
- Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74(15):4042–4052.
- Mkrtichyan M, et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15.View this article via: PubMed Google Scholar
- Wada S, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89.View this article via: PubMed Google Scholar
- Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17(8):2270–2280.
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19(5):1035–1043.
- Cuadros C, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116(6):934–943.
- Ito F, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64(22):8411–8419.
- Melero I, Martinez-Forero I, Dubrot J, Suarez N, Palazon A, Chen L. Palettes of vaccines and immunostimulatory monoclonal antibodies for combination. Clin Cancer Res. 2009;15(5):1507–1509.
- Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19(5):1009–1020.
- Curti BD, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198.
- He LZ, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191(8):4174–4183.
- Wei SM, et al. Anti-CD27 antibody potentiates antitumor effect of dendritic cell-based vaccine in prostate cancer-bearing mice. Int Surg. 2015;100(1):155–163.
- Bos R, et al. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 2008;68(20):8446–8455.
- Newton MR, et al. Anti-interleukin-10R1 monoclonal antibody in combination with bacillus Calmette — Guerin is protective against bladder cancer metastasis in a murine orthotopic tumour model and demonstrates systemic specific anti-tumour immunity. Clin Exp Immunol. 2014;177(1):261–268.
- Du S, Barcellos-Hoff MH. Tumors as organs: biologically augmenting radiation therapy by inhibiting transforming growth factor beta activity in carcinomas. Semin Radiat Oncol. 2013;23(4):242–251.
- Fabregat I, Fernando J, Mainez J, Sancho P. TGF-β signaling in cancer treatment. Curr Pharm Des. 2014;20(17):2934–2947.
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nat Rev Immunol. 2010;10(8):554–567.
- Milagre CS, et al. Adaptive upregulation of EGFR limits attenuation of tumor growth by neutralizing IL-6 antibodies with implications for combined therapy in ovarian cancer. Cancer Res. 2015;75(7):1255–1264.
- Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2(4):288–294.
- Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014;25(4):377–390.
-
Version history
- Version 1 (July 27, 2015): No description
- Version 2 (July 27, 2015): No description
- Version 3 (September 1, 2015): No description
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Review Series
Cancer Immunotherapy
-
Therapeutic cancer vaccines
Cornelis J.M. Melief et al. -
From mice to humans: developments in cancer immunoediting
Michele W.L. Teng et al. -
Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected
Douglas Marvel et al. -
Tumor neoantigens: building a framework for personalized cancer immunotherapy
Matthew M. Gubin et al. -
Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages
Stefano Ugel et al. -
Immunity, inflammation, and cancer: an eternal fight between good and evil
Shabnam Shalapour et al. -
Cancer immunotherapy: harnessing the immune system to battle cancer
Yiping Yang -
CAR therapy: the CD19 paradigm
Michel Sadelain -
Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future
Lieping Chen et al. -
Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade
Elizabeth Buchbinder et al.
Metrics



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)





