Advertisement
Review Series Free access | 10.1172/JCI80007
Immunity, inflammation, and cancer: an eternal fight between good and evil
Shabnam Shalapour and Michael Karin
Laboratory of Gene Regulation and Signal Transduction, Departments of Pharmacology and Pathology, School of Medicine, UCSD, San Diego, California, USA.
Address correspondence to: Michael Karin, UCSD, 9500 Gilman Drive, MC#0723, La Jolla, California 92093, USA. Phone: 818.534.1361; E-mail: karinoffice@ucsd.edu.
Find articles by Shalapour, S. in: PubMed | Google Scholar
Laboratory of Gene Regulation and Signal Transduction, Departments of Pharmacology and Pathology, School of Medicine, UCSD, San Diego, California, USA.
Address correspondence to: Michael Karin, UCSD, 9500 Gilman Drive, MC#0723, La Jolla, California 92093, USA. Phone: 818.534.1361; E-mail: karinoffice@ucsd.edu.
Find articles by Karin, M. in: PubMed | Google Scholar
Published September 1, 2015 - More info
J Clin Invest. 2015;125(9):3347–3355. https://doi.org/10.1172/JCI80007.
Copyright © 2015, American Society for Clinical Investigation
-
Abstract
Cancer development and its response to therapy are strongly influenced by innate and adaptive immunity, which either promote or attenuate tumorigenesis and can have opposing effects on therapeutic outcome. Chronic inflammation promotes tumor development, progression, and metastatic dissemination, as well as treatment resistance. However, cancer development and malignant progression are also associated with accumulation of genetic alterations and loss of normal regulatory processes, which cause expression of tumor-specific antigens and tumor-associated antigens (TAAs) that can activate antitumor immune responses. Although signals that trigger acute inflammatory reactions often stimulate dendritic cell maturation and antigen presentation, chronic inflammation can be immunosuppressive. This antagonism between inflammation and immunity also affects the outcome of cancer treatment and needs to be considered when designing new therapeutic approaches.
-
Introduction
Inflammation has been recognized since the beginning of recorded medical knowledge (1–3). It is a part of a complex biological response to cellular damage caused either by sterile injury (cell death) or infection, in which the immune system attempts to eliminate or neutralize injurious stimuli and initiates healing and regenerative processes. For example, IL-6, a key tumor-promoting inflammatory cytokine produced by innate immune cells, activates at least three regeneration-promoting transcription factors — YAP, Notch, and STAT3 — which are also involved in stem cell activation (4). It is likely that all tumor-promoting inflammation, whether it precedes or follows tumor development, is part of the normal response to injury and infection that has been usurped by cancer cells to their own advantage.
Inflammation is classically viewed as a feature of innate immunity, which differs from adaptive immunity by the receptors mediating its activation and its rapid onset. Innate immunity is also more evolutionarily ancient than adaptive immunity and is triggered by foreign microbial and viral structures, known as pathogen-associated molecular patterns (PAMPs), or normal cellular constituents released upon injury and cell death, known as damage-associated molecular patterns (DAMPs). Both PAMPs and DAMPs are recognized by pattern-recognition receptors (PRRs), many of which belong to the TLR family (5, 6). Once activated, innate immunity results in upregulation of MHC class I and II and costimulatory molecules, as well as numerous inflammatory chemokines and cytokines that attract and prime T cells for activation through diverse antigen receptors (7). Activated adaptive immune cells, including T and B lymphocytes, further amplify the initial inflammatory response. Thus, type 1 helper T cells (Th1 cells) activate macrophages both through cell-to-cell contact and IFN-γ secretion (8), Th2 cells activate eosinophils through cytokine release, and B cells secrete antibodies that activate the complement cascade — as well as phagocytes, NK cells, and mast cells — through Fc receptors (7, 9–12). However, certain adaptive immune cells, especially Tregs, can turn off the inflammatory response (13).
The major driving forces that contribute to evolution of the immune system are infectious organisms capable of eliciting direct damage to the host. Yet, despite its sophistication, the immune system can cause substantial collateral damage (immunopathology) when over-activated or not properly terminated. To minimize immunopathology and maximize host defense, innate and adaptive immune cells are equipped with negative regulatory mechanisms (14–18). In fact, maximal immunity is achieved only when innate and adaptive immune cells act in concert and harmony, which also depends on negative control or immunosuppressive mechanisms. For instance, during chronic viral infections, viruses are held at bay while avoiding immunopathological damage by immune checkpoints that prevent an overzealous antiviral response (19). These evolutionarily conserved controls may also be involved in T cell tolerization during cancer-associated chronic inflammation (20, 21), although the underlying mechanisms remain obscure (22–24). In this review, we will discuss how innate and adaptive immune cells control tumor progression and the response to therapy, and we will try to avoid extensive discussion of the entire inflammation and cancer field, which has been reviewed elsewhere (20, 25, 26).
-
The evil: chronic inflammation and cancer
The first documented proposition of an association between inflammation and cancer has been attributed to the German pathologist Rudolf Virchow, who was active in the mid-19th century. This hypothesis, based on Virchow’s detection of inflammatory infiltrates in solid malignancies, has gained strong epidemiological and mechanistic support in the past dozen years (20), leading to recognition of tumor-associated inflammation as a key feature (hallmark) of cancer (20, 27, 28). While early work has mainly addressed the link between preexisting inflammation and subsequent tumor development, which may account for 15%–20% of cancer deaths (25), more recent efforts have been dedicated to understanding tumor-elicited inflammation, the inflammatory reaction that follows tumor development and is detected in nearly all solid malignancies.
One of the best-studied cancers from a genetic perspective has been colorectal cancer (CRC), where the majority of cases follow a well-charted genetic pathway in which premalignant lesions, called advanced crypt foci (ACF), are formed as a result of β-catenin activation, mainly due to loss of the antigen-presenting cell (APC) tumor suppressor (29). Additional K-Ras activating mutations lead to formation of adenomas, which progress to invasive carcinomas upon loss of p53 and components of the TGF-β signaling pathway (30). The elucidation of this process led to the view that cancer is a genetic disease in which environmental factors come into play solely through induction of new somatic mutations. For instance, chronic inflammation due to inflammatory bowel disease (IBD), which increases CRC risk, was thought to act mainly through production of mutagenic ROS and reactive nitrogen species (RNS) (30). Although expression of inducible NO synthase (iNOS) induces oxidative DNA damage and accelerates loss of heterozygosity at the Apc locus to contribute to CRC induction (31), IBD promotes CRC development mainly through activation of NF-κB and STAT3 (32, 33), and perhaps YAP and Notch (4), transcription factors that activate genes that promote the survival of initiated epithelial cells and expose them to growth-promoting inflammatory cytokines (30). More recently, it became clear that even without preexisting IBD, inflammation occupies a key position in the development of sporadic CRC. As soon as the Apc locus is lost in mice and ACF lesions appear, there is an accompanying loss of mucin2 production and junctional adhesion molecules, resulting in barrier defects and invasion of ACF lesions and early adenomas with commensal enteric bacteria or their products (34). The latter activate nearby macrophages through TLR2, -4, and -9 to secrete IL-23, which stimulates the production of IL-17A through its effects on Th17 cells and innate lymphoid cells (34). IL-17A, in turn, directly stimulates the proliferation and growth of ACF lesions into adenomas and adenocarcinomas (35). Early human adenomas also exhibit loss of the epithelial barrier, microbial invasion, and upregulation of IL-23 and IL-17A (34). Furthermore, elevated expression of IL-17A and IL-23R in stage I and II human CRC correlates with rapid progression to lethal metastatic disease (36). Moreover, IL-11, an IL-6 family member mainly produced by myeloid cells and cancer-associated fibroblasts (CAFs), also supports tumor promotion and progression by activating gp130/STAT3 signaling in gastrointestinal cancers (37, 38). Another cytokine, IL-22 — an IL-10 family member that is mainly secreted by T cells, innate lymphoid cells, and DCs — also acts through STAT3 to promote tissue repair and tumorigenesis in colon and liver (39–41). In addition, IL-6 and related cytokines are induced upon IL-17R rengagement and may enhance tumor progression at later stages (35). Neutralization of IL-17A, IL-11, or IL-22 can inhibit colonic tumorigenesis at an early stage, underscoring the fundamental importance of tumor-elicited inflammation in the malignant progression of colorectal tumors. Such findings and others counter the recent suggestion that cancer rates and risks are purely dictated by the number of cancer-initiating cell divisions (42). We suggest, instead, that the key rate-limiting step in cancer development is the progression of premalignant lesions, many of which (excluding ACF lesions) can exist in a dormant state for years before becoming malignant growths. This step is controlled both by intrinsic (tumor-elicited) and extrinsic inflammation, and may be attenuated by antitumor immunity, which was suggested to maintain dormancy (23).
-
The good: immunity and cancer
In recent years, tumor immunologists and practicing oncologists have seen a dream come true with the clinical implementation and regulatory approval of cancer immunotherapies (43). It was first suggested by Paul Ehrlich, 50 years after Virchow, that the immune system can fight tumors (44), a suggestion reiterated by the immunosurveillance hypothesis of Burnet and Thomas (45). These hypotheses are based on the notion that cancer-associated genetic alterations, together with aberrant quality-control mechanisms and epigenetic reprogramming, result in expression of tumor-specific antigens (neoantigens) and tumor-associated antigens (TAAs), which are nonmutated proteins to which T cell tolerance is probably incomplete due to their restricted tissue expression pattern (46). These antigens can activate antitumor immunity and, under certain circumstances, may also induce rejection of early neoplasms, a concept known as immunosurveillance (23, 46). However, the tumor-controlling ability of the immune system was not widely accepted until the successful development of immune checkpoint inhibitors that trigger tumor rejection by activating cytotoxic T lymphocytes (CTLs). The response to such immunotherapeutics and their clinical benefit were shown to depend on the intrinsic mutational rate for the cancer being treated (47). However, it is still not clear which type of neoantigen induces a better protective immunity, as tumors with immunogenic passenger mutations are most likely to develop resistance, compared with those with immunogenic driver mutations (48, 49). Based on these data, one would assume that early neoplasms might have too few mutations to be recognized by our T cells. Furthermore, established tumors with higher mutation rates use various escape mechanisms to bypass immunosurveillance, including immunoediting, antigen loss variants, MHC downregulation (23), and induction of immunogenic tolerance (24, 50–52). Tolerance induction can be achieved by production of negative regulatory signals and recruitment of immunosuppressive cells to the tumor microenvironment (24, 50, 52). In addition to Tregs, this population of immunosuppressive cells includes so-called myeloid-derived suppressor cells (MDSCs), regulatory B cells (Bregs), and immunosuppressive plasmocytes (ISPC) (24, 51–53).
The recent success of immune checkpoint inhibitors in the treatment of melanoma, non–small cell lung carcinoma (NSCLC), and bladder and kidney cancers suggests that a fraction of these cancers still display or release sufficient amounts of potent tumor antigens. It is the elevated expression of negative regulatory signals and the presence of immunosuppressive cells that account for establishment of immune tolerance in such cancers. However, while immune checkpoint blockade and adoptive T cell transfer strategies can result in a clinical benefit in a subset of patients, most patients are still refractory to such therapies (43, 54, 55). Thus, future effort should be directed toward increasing response rates and expanding the applicability of immunotherapy to all types of cancer.
We will discuss how tumor-related chronic inflammation shapes local and systemic innate and adaptive immunity to promote formation of an immunosuppressive tumor microenvironment, especially during cancer therapy. We also review the dual roles played by different immune cell types in promoting tumor inflammation or immunity, and progression or regression.
-
Innate immune cells: the good APC and the evil TAM, TAN, and MDSC
Myeloid cells, including macrophages and neutrophils, are the most abundant immune cells in the tumor microenvironment (56, 57). Tumor-associated macrophages (TAMs) acquire protumorigenic properties in primary and metastatic sites, and they play supportive roles in cancer development and progression by stimulating cell proliferation, cell survival, angiogenesis, invasive and motile behavior, and suppression of CTL responses (56, 58–60). At early stages of tumor development, TAMs appear to undergo classical activation and exhibit an M1 phenotype (referred to as TAM1) (61), rather than the alternatively activated M2 phenotype (TAM2), which may form as tumors accumulate lactic acid and acquire hypoxic cores (62). Exposure of macrophages to IL-4 produced by CD4+ T cells and/or cancer cells (59, 63), growth factors such as colony stimulating factor-1 (CSF1) (64), GM-CSF (65), and TGFβ secreted by cancer cells, can also induce the M2 switch. It remains to be determined which TAM type is more important in tumor promotion, but it should be noted that TAM1 cells express and secrete classical proinflammatory cytokines, chemokines, and effector molecules, including IL-1, IL-6, TNF, IL-23, and iNOS, which are known to contribute to tumor initiation and early promotion (20). TAM2 cells produce VEGF and antiinflammatory molecules, such as IL-10, TGF-β, and arginase 1 (ARG1) (57, 60). The blood vessels that provide oxygenation and nutrition dramatically increase in most tumors during malignant conversion, a process known as the angiogenic switch, and a similar process may occur during cancer therapy. TAMs that express TIE2 regulate this process mostly via production of VEGF and are mainly considered to be TAM2 cells (66, 67). TIE2+ macrophages also promote cancer cell migration and intravasation (68), which come into play during late stages of tumor progression. Based on such observations, we propose that TAM2 cells may be more important in later stages of tumor growth, which depend on angiogenesis and immunosuppression. Macrophages are plastic in nature and easily alter their gene expression program during tumor progression rather than assume irreversible fates. Additionally, TAM2 cells express membrane-bound or soluble forms of HLA molecules that can directly inhibit activation of NK cells and certain T cell subsets (69). TAMs can also express programmed death-1 ligand (PD-L1) upon activation of HIF-1α in hypoxic tumor regions, further inhibiting CTL activation (70). However, tumors lacking T cell–based inflammation may require innate immune cells to promote T cell recruitment and activation (50, 71, 72).
Tumor-associated neutrophils (TANs) exhibit both antitumoral and protumoral functions. TANs can mediate cancer cell killing and promote metastasis by releasing ROS and neutrophil elastase, and potentiate antitumoral T cell responses by inhibiting TGF-β signaling (66). TANs promote genetic instability through ROS and RNS release; sustain angiogenesis by releasing VEGF, MMP-9, or prokineticin 2 (Bv8); and enhance neoplastic cell invasiveness through soluble mediators (e.g., oncostatin M [OSM] and HGF) (56, 73, 74).
MDSCs are an ill-defined population of cells that express markers of monocytes, macrophages, and neutrophils (75). Most of their immunosuppressive activities are similar to those of TAM2 cells and TANs, and it may be difficult to distinguish between these cells, since they express several common markers. Furthermore, immunosuppression in the tumor bed is primarily mediated by TAMs and TANs that contribute to the inhibition of CTL (60), and it is not clear whether MDSCs are a truly distinct population or immature inflammatory monocytes that were recently recruited into the tumor.
DCs, the main type of professional APCs, play an important role in T cell priming. The generation of protective antitumor immunity depends on DC maturation and antigen presentation (72, 76). DC and macrophages express MHC class I molecules, which present antigens to CD8+ T cells. Host type 1 IFN signals are required for mounting an antitumor CTL response through CD8α+ DCs. Notably, some conventional chemotherapeutics, when administered at a low dose, and certain forms of radiation therapy induce tumor rejection through immunogenic cell death (ICD), which depends on the release of DAMPs and antigens (77–79). The DAMPs bind to receptors expressed on the surface of APCs and stimulate their maturation and ability to present TAAs through an acute proinflammatory pathway (77). Such APCs acquire the ability to support cancer-specific immune responses and promote tumor regression (80). Based on this knowledge, DC-based therapeutic vaccination has been developed, but so far only a modest transient response has been observed (81–83).
-
Janus-faced innate lymphocytes: NK, NKT, and γδ T cells
NK cells, natural killer T (NKT) cells, and γδ T cells populate many tumors (84–86). NK cells recognize mouse cancer cells via ribonucleic acid export–1 (RAE-1) family ligands and human cancer via MHC class I–related genes A and B (MICA and MICB), all of which bind the NK cell–activating receptor NKG2D (84, 87, 88). These NKG2D ligands are upregulated during the DNA damage response and cell cycle progression via E2F transcription factors (89). The antitumor activity of NK cells was mainly observed in hematopoietic malignancies, but it was recently shown that in mice, MULT1, a high-affinity NKG2D ligand that is released by cancer cells, causes NK cell activation and rejection of solid tumors (90). NK cells have a dual role during liver inflammation and injury, where they contribute to both antiviral defense and tumor-promoting tissue damage. NK cells control liver fibrosis by killing early or senescence-activated hepatic stellate cells and produce antifibrogenic IFN-γ (91). However, CD8+ T cells and NKT cells also promote nonalcoholic steatohepatitis (NASH) and accelerate its progression to hepatocellular carcinoma (HCC) (92). CD1d-restricted NKT cells have both innate and adaptive characteristics (93), and a subset of NKT cells was reported to suppress antitumor immunity, in part via production of IL-13, which in turn induces TGF-β production by myeloid cells (94). γδ T cells also have dual functions; they are antitumorigenic after chemotherapy (95, 96) and immunosuppressive in isolated breast cancer tissue (97). IL-17 expression from γδ T cells was shown to induce a T cell–suppressive TAN phenotype in mice bearing mammary tumors that promote metastatic spread (98).
-
CAFs: underappreciated immune regulators
Inflammatory responses are often accompanied by recruitment of fibroblasts and induction of fibrosis. CAFs are responsible for deposition of collagen and various extracellular matrix components (ECM components) in the tumor microenvironment, where they stimulate cancer cell proliferation and angiogenesis (99, 100). CAFs also have a critical but underappreciated immune function as they produce numerous cytokines and chemokines, including osteopontin (OPN), CXCL1, CXCL2, IL-6, IL-1β, CCL-5, stromal-derived factor-1α (SDF-1α), and CXCL13 (101, 102). During early tumorigenesis, fibroblasts sense changes in tissue architecture caused by increased proliferation of neighboring epithelial cells and respond to these changes by producing proinflammatory mediators (103). The proinflammatory properties of CAFS are enhanced by mediators secreted by resident immune cells (102), such as IL-1, which activates Fc receptors (104). CAFs are also activated during therapy-induced hypoxia and produce copious amounts of TGF-β and numerous chemokines, including B cell–recruiting CXCL13 (105). CAF-secreted CCL2 recruits macrophages to the tumor microenvironment (106), whereas CAF-derived TGF-β inhibits NK cell and CTL activation, and induces Treg and ISPC differentiation (53, 107). CAF-derived CXCL13 mediates recruitment of B cells into androgen-deprived prostate cancer, leading to development of hormone resistance (105, 108). Activated CAFs expressing fibroblast activation protein-α (FAP) were also reported to suppress antitumor immunity in Lewis lung carcinoma (109). In contrast, in a mouse model of pancreatic cancer, depletion of activated αSMA+ CAFs induced immunosuppression that correlated with increased recruitment of CD4+Foxp3+ Tregs (110). Moreover, mesenchymal stem cells (MSC), which are multipotent stromal cells that can give rise to CAFs (111), exist in many tissues, especially tumors, and their contribution to tissue regeneration and modulation of inflammation has been described (112, 113). MSCs suppress immunity in some cases and enhance it in others by producing factors like TGF-β; indoleamine 2,3-dioxygenase (IDO); IL-7; or IL-15 (113, 114). MSCs and CAFs also secrete IL-6 and orchestrate lymphoid tissue growth and responses (115).
-
Dual roles of T cell subsets in cancer
Many tumors express antigens that can be recognized by T lymphocytes, and analysis of the tumor microenvironment often reveals T cell infiltrates (116). Although CD8+ T cells are generally antitumorigenic, CD4+ T cell subpopulations can either promote or inhibit tumor progression. For example, CD4+Foxp3+ Tregs play a pivotal role in maintenance of immunological tolerance (117) and also produce cytokines, such as RANKL, that promote breast cancer progression and metastasis (118). Th17 cells produce IL-17A and IL-17F, which accelerate CRC progression (discussed above, ref. 34) but may also possess an antitumorigenic function in other malignancies (119, 120).
CTL activation involves DAMP release upon ICD, which promotes DC maturation and antigen presentation (53, 77, 121). Despite an active CTL response in a subset of patients, established tumors that progress are obviously not rejected. This indicates the existence of potent immunosuppressive mechanisms that neutralize antitumor immunity, including induction of an exhausted/anergic-like CD8+ T cell phenotype, which may be a product of ongoing cancer-associated inflammation, as well as chronic inflammation caused by multiple rounds of cancer therapy. After acute infection, naive, antigen-specific CD8+ T cells become activated, proliferate, and acquire effector functions. After pathogen clearance, 5%–10% of effector CD8+ T cells survive and differentiate into memory cells (122). During persistent infections, chronic inflammation, and tumor development, antigen-specific CD8+ T cells often fail to form effector or memory cells (24, 123) and instead undergo exhaustion, as first described during viral infection (124). Importantly, CD8+ T cell effector functions are lost in a hierarchical manner during chronic inflammation, with some functions exhausted earlier (e.g., IL-2 production, cytotoxicity, and proliferation) than others (e.g., IFN-γ) (125). Anergic/exhausted CD8+ T cells accumulate when antigen load is high and CD4+ T cell help is lacking (126).
Multiple regulatory pathways were shown to mediate T cell exhaustion in a context-dependent manner. The inhibitory receptor PD-1 is an important regulator of virus- and cancer-specific CD8+ T cell exhaustion during chronic inflammation in mice, primates, and humans (127, 128). IL-10 was also implicated in CTL dysfunction (129). Additional pathways, including CTL antigen 4 (CTLA-4), seem to influence CTL function and differentiation during persistent chronic inflammation and cancer. Importantly, tolerance and exhaustion are not irreversible fates, and depending on external conditions, anergic/exhausted cells can be reprogrammed and their immune effector function can be resumed. Many of the factors involved in T cell suppression are expressed or secreted by cancer- and tumor-associated cells, including IL-10, TGF-β, iNOS, ROS, and IDO. Tumor-specific CD8+ T cells showed an exhausted phenotype not only in primary tumors but also in metastases (130, 131). Furthermore, ZEB1, an epithelial-to-mesenchymal-transition (EMT) activator, increased PD-L1 expression on cancer cells, thereby causing CD8+ T cell exhaustion, which promotes metastasis (132). Curiously, upregulation of PD-L1, IDO, and Tregs in the melanoma microenvironment may be driven by CD8+ T cells (133). We found that activation and infiltration of ISPCs requires the presence of CD8+ T cells, even though ISPCs eventually suppress CTL activation (53). Chemotherapy-induced ICD results in tumor infiltration with CD8+ T cells, but these cells exhibit an anergic/exhausted phenotype unless ISPCs are removed from the tumor bed through genetic or immunological manipulations (53). Inflammation-induced resistance to immunotherapy was also described in melanomas, which acquire resistance to adoptive T cell therapy (ATCT) through TNF-dependent loss of melanocytic antigens and IFN-γ–dependent PD-L1 expression (134). These data suggest that the majority of observed immunosuppressive pathways, whose molecular nature is discussed below, are intrinsically driven by the immune system rather than being orchestrated by cancer cells, and may be driven by the negative feedback mechanisms that limit an overactive immune response. These are the remnants of the ancient protective mechanisms that had evolved to limit collateral damage during eradication of viral and microbial infections.
-
Good and bad B cells
Immunoglobulins specific for more than 2,000 antigens, which are often overexpressed by cancer cells, were detected in the sera of cancer patients (135, 136); however, antibody-mediated cancer cell killing is impaired. Notably, antibody effector functions that are mediated by Fcγ receptors are also compromised during persistent infections, an effect attributed to formation of antigen/antibody immune complexes (ICs), suggesting that high concentrations of preexisting ICs can limit the effectiveness of antibody therapy in human cancer (137). Moreover, certain immunoglobulins exhibit an antitumorigenic function (138). B lymphocytes are also present in the tumor microenvironment and in mouse models of squamous cell carcinoma, where they promote progression by activating mast cells and other myeloid cells (139). In prostate cancer, however, newly recruited B cells promote aggressive hormone-resistant tumors by producing the proinflammatory cytokine lymphotoxin (108). Recently, B cells including Bregs and ISPCs were shown to attenuate the development of autoimmune disease (140) and antitumor immunity (53, 141–143). We found a subpopulation of ISPCs that produce IgA, PD-L1, and IL-10 and strongly inhibit CTL activation in prostate cancer–bearing mice treated with the immunogenic chemotherapeutic agent oxaliplatin (53). These cells are also present in human prostate cancer, especially in treatment refractory and metastatic tumors. Unlike B cells in skin cancer (143), ISPCs in prostate cancer directly inhibit CTL activation by expressing IL-10 and PD-L1 (53). Elimination of ISPCs, whose development depends on TGF-β signaling, strongly enhances the immunogenic response to low-dose oxaliplatin and results in tumor rejection (53).
-
The molecular “Yin-Yang” of cancer inflammation and immunity
Although induction of T cell exhaustion through inhibitory receptors like PD-1, TIM-3, LAG3, CTLA-4, and BTLA has been extensively described (144), little is known about the factors that regulate receptor and ligand expression in chronically inflamed tissues or tumors. So far, STAT3, STAT4, and SMAD transcription factors, which are also involved in regulation of chronic inflammation (20), seem to control expression of most of these inhibitory receptors (144). These factors are activated by TGF-β and IL-10, both of which are present in the tumor microenvironment. TGF-β, for instance, is a key regulator of the wound-healing and fibrotic response. Its expression in CAFs is induced by tumor hypoxia that occurs either in response to O2 shortage during rapid tumor growth or blood vessel collapse induced by therapy (105). TGF-β may suppress CTL activation through SMAD activation and direct binding of SMAD/activating transcription factor-1 (ATF1) complexes to the granzyme B and IFNG promotor regions (145). TGF-β also suppresses CTL activation indirectly by inducing regulatory cells including Tregs, ISPCs, and tolerogenic APCs (53, 146, 147). In this context, TGF-β signaling may synergistically interact with IL-6 or IL-10 through STAT3. We found that TGF-β signaling in combination with STAT3, which may be activated by IL-21 and autocrine IL-10, induces formation of IgA+ ISPCs through class-switch recombination (53). Galectin-9, a TIM-3 ligand, promotes TGF-β–dependent induction of Tregs by activating SMAD and ERK (147). Moreover, activation of STAT3 together with ERK or p38 induces IL-10 expression in B cells (148). In addition to suppressing CTL effector functions, IL-10 induces tolerogenic macrophages and APCs by elevating their PD-L1 expression (149). PD-L1 expression by MDSCs is another direct target for HIF-1α (70), which is responsible for CAF activation in prostate cancer (105).
-
Conclusions and prospects
In addition to its well-studied effects on cancer cell proliferation and survival, tumor-associated inflammation also plays an important role in the suppression of antitumor immunity (Figures 1 and 2). As discussed above, the immunosuppressive mechanisms triggered by tumor-associated inflammation are just beginning to be elucidated. Clearly, further investigation into this new crosstalk between innate and adaptive immunity will facilitate the development of new therapeutic strategies that boost antitumor immunity by targeting immunosuppressive chronic inflammation. In addition to combining immunogenic chemotherapeutics that induce tumor antigen release and presentation with immune checkpoint inhibitors, it should be possible to identify small molecules or neutralizing antibodies that inhibit the induction, accumulation, and function of immunosuppressive cell types in response to cancer-associated inflammation. All of these manipulations may prevent CTL exhaustion and support antitumor immunity. The availability of such reagents is likely to expand the new era in cancer therapy brought about by immune checkpoint inhibitors.
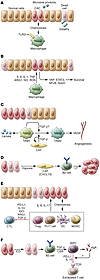 Figure 1
Figure 1Inflammation promotes tumor development. (A) Microbial products that penetrate through the defective barrier associated with early tumors or DAMPs released by dying cancer cells (CACs) activate myeloid cells that are recruited into the tumor due to production of chemokines by CACs. (B) TAMs and TANs express cytokines, such as IL-1, IL-6, and TNF, which act directly on CACs, leading to activation of NF-κB, STAT3, YAP, and Notch. The cytokines thereby promote CAC survival and proliferation. (C) The growing tumor secretes lactate and acquires a hypoxic core due to insufficient O2 supply. The hypoxia results in CAF activation due to HIF-1–induced TGF-β production and may convert TAM1 cells to a TAM2 phenotype, which produces VEGF to support neo-angiogenesis. (D) CAFs, which express TGF-β and CXCL13, recruit lymphotoxin-producing B2 cells that support further tumor growth. (E) Chemokines expressed in the inflamed tumor bed recruit tumor-promoting Th17 cells and immunosuppressive Tregs and MDSCs. (F) Tumor-infiltrating B cells undergo class-switch recombination (CSR) and become ISPCs that induce an exhausted/angergic-like phenotype in cytotoxic T cells.
 Figure 2
Figure 2Acute inflammation promotes antitumor immunity in response to immunogenic chemotherapy in noninflamed tumors. ICD is induced by injury, stress, and certain chemotherapeutic agents. ICD can induce expression of surface calreticulin and HMGB1 in cancer cells, thereby activating innate immune cells through PRRs. DC maturation and antigen cross-presentation, together with secretion of inflammatory cytokines, can efficiently prime cytotoxic T cells, resulting in effective antitumor immune responses. However, in tumors with a chronically inflamed microenvironment rich in immunosuppressive factors, antitumor immunity cannot be activated unless the immunosuppressive factors are neutralized or eliminated.
-
Acknowledgments
Research in our laboratory was supported by the NIH (CA127923 and AI043477) and postdoctoral research fellowships from the German Research Foundation and Irvington-Cancer research Institute (to S. Shalapour). M. Karin is an American Cancer Society Research Professor and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases.
Address correspondence to: Michael Karin, UCSD, 9500 Gilman Drive, MC#0723, La Jolla, California 92093, USA. Phone: 818.534.1361; E-mail: karinoffice@ucsd.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2015;125(9):3347–3355. doi:10.1172/JCI80007.
-
References
- Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2014;14(1):51–59.View this article via: PubMed Google Scholar
- Spencer WG. Celsus. De medicina. Loeb Classical Library. Cambridge, Massachusetts, USA: Harvard University Press; 1938.
- Nunn JF. Ancient Egyptian Medicine. Norman, Oklahoma, USA: University of Oklahoma Press; 2002.
- Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62.
- Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290.
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9(10):692–703.
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22.
- Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541.
- Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–494.
- Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14(2):94–108.
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353.
- Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37(2):199–207.
- Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123(3):939–944.
- Kim KD, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13(10):1248–1252.
- Xu S, et al. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat Immunol. 2012;13(6):551–559.
- Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320(5880):1220–1224.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594.
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899.
- Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35(4):467–477.
- Speiser DE, Utzschneider DT, Oberle SG, Munz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014;14(11):768–774.
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25.
- Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60.View this article via: PubMed Google Scholar
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444.
- Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706.
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–436.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–799.
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114 e2105.
- Shaked H, et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci U S A. 2012;109(35):14007–14012.
- Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113.
- Greten FR, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296.
- Grivennikov SI, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258.View this article via: PubMed Google Scholar
- Wang K, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41(6):1052–1063.
- Tosolini M, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–1271.
- Putoczki TL, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24(2):257–271.
- Necula LG, et al. IL-6 and IL-11 as markers for tumor aggressiveness and prognosis in gastric adenocarcinoma patients without mutations in Gp130 subunits. J Gastrointestin Liver Dis. 2012;21(1):23–29.View this article via: PubMed Google Scholar
- Zhang W, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14(20):6432–6439.
- Huber S, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491(7423):259–263.
- Jiang R, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54(3):900–909.
- Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81.
- Schumacher TN, Kesmir C, van Buuren MM. Biomarkers in cancer immunotherapy. Cancer Cell. 2015;27(1):12–14.
- Ehrlich P. The current state of cancer research (Über den jetzigen stand der karzinomforschung). Ned Tijdschr Geneeskd. 1909;5:273–290.
- Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27.
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74.
- Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199.
- Snyder A, Chan TA. Immunogenic peptide discovery in cancer genomes. Curr Opin Genet Dev. 2015;30C:7–16.View this article via: PubMed Google Scholar
- van Rooij N, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31(32):e439–e442.
- Gajewski TF, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25(2):268–276.
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296.
- Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 2015;75(1):5–10.
- Shalapour S, et al. Immunosuppressive plasma cells impede T cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–98.
- Galluzzi L, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5(24):12472–12508.View this article via: PubMed Google Scholar
- Dudley ME, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31(17):2152–2159.
- Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–1410.
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51.
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291.
- Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637.
- Franklin RA, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925.
- Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563.
- Gocheva V, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241–255.
- Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7(2):147–162.
- Su S, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620.
- Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20.
- Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62(23):7042–7049.View this article via: PubMed Google Scholar
- Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064–5066.
- Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187(5):813–818.
- Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790.
- Ji RR, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–1031.
- Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–2016.
- Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9(9):1732–1737.
- Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–8904.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174.
- Broz ML, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–652.
- Ma Y, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741.
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
- Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474.
- Cirone M, et al. Activation of dendritic cells by tumor cell death. Oncoimmunology. 2012;1(7):1218–1219.
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48.
- Pizzurro GA, Barrio MM. Dendritic cell-based vaccine efficacy: aiming for hot spots. Front Immunol. 2015;6:91.View this article via: PubMed Google Scholar
- Carreno BM, et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. [published online ahead of print April 2, 2015]. Science. doi:10.1126/science.aaa3828.
- Guerra N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–580.
- Kelly JM, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3(1):83–90.
- Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–5943.
- Mishra R, Chen AT, Welsh RM, Szomolanyi-Tsuda E. NK cells and gammadelta T cells mediate resistance to polyomavirus-induced tumors. PLoS Pathog. 2010;6(5):e1000924.
- Fine JH, et al. Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer Res. 2010;70(18):7102–7113.
- Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med. 2012;209(13):2409–2422.
- Deng W, et al. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348(6230):136–139.
- Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832(7):1061–1069.
- Wolf MJ, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–564.
- Robertson FC, Berzofsky JA, Terabe M. NKT cell networks in the regulation of tumor immunity. Front Immunol. 2014;5:543.View this article via: PubMed Google Scholar
- Terabe M, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520.
- Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56(8):1285–1297.
- Ma Y, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208(3):491–503.
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–348.
- Coffelt SB, et al. IL-17-producing γΔ T cells and neutrophils conspire to promote breast cancer metastasis. [published online ahead of print March 30, 2015]. Nature. doi:10.1038/nature14282.
- Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348.
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337.
- Chang HY, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2(2):E7.
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell. 2010;17(2):135–147.
- Wong VW, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18(1):148–152.View this article via: PubMed Google Scholar
- Andreu P, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–134.
- Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci U S A. 2014;111(41):14776–14781.
- Ksiazkiewicz M, Gottfried E, Kreutz M, Mack M, Hofstaedter F, Kunz-Schughart LA. Importance of CCL2-CCR2A/2B signaling for monocyte migration into spheroids of breast cancer-derived fibroblasts. Immunobiology. 2010;215(9):737–747.View this article via: PubMed Google Scholar
- Yang L, Pang Y, Moses HL. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–227.
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–305.
- Kraman M, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science. 2010;330(6005):827–830.
- Ozdemir BC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734.
- Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–272.
- Scheel C, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940.
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016.
- Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843.
- Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110(2):469–478.
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39(1):11–26.
- Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210(11):2435–2466.
- Tan W, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553.
- Xie Y, Sheng W, Xiang J, Ye Z, Yang J. Interleukin-17F suppresses hepatocarcinoma cell growth via inhibition of tumor angiogenesis. Cancer Invest. 2010;28(6):598–607.
- Viaud S, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976.
- Weiner LM, Lotze MT. Tumor-cell death, autophagy, and immunity. N Engl J Med. 2012;366(12):1156–1158.
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78(11):5535–5545.
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19(4):408–415.
- Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213.
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927.
- Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22(2):223–230.
- Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–14738.
- Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010;134(3):237–250.
- Ng CT, Oldstone MB. IL-10: achieving balance during persistent viral infection. Curr Top Microbiol Immunol. 2014;380:129–144.View this article via: PubMed Google Scholar
- Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360.
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86.
- Chen L, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241.
- Spranger S, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116.View this article via: PubMed Google Scholar
- Landsberg J, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490(7420):412–416.
- Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12(4):307–313.
- Schreiber H, Ward PL, Rowley DA, Stauss HJ. Unique tumor-specific antigens. Annu Rev Immunol. 1988;6:465–483.
- Wieland A, et al. Antibody effector functions mediated by Fcγ-receptors are compromised during persistent viral infection. Immunity. 2015;42(2):367–378.
- Baker K, et al. Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer. Immunity. 2013;39(6):1095–1107.
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423.
- Shen P, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–370.
- Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4(5):627–630.
- Olkhanud PB, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–3515.
- Affara NI, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–821.
- Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33C:23–35.View this article via: PubMed Google Scholar
- Thomas DA, Massague J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–380.
- Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453.
- Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 promotes TGF-β1-dependent induction of regulatory T cells via the TGF-β/Smad signaling pathway. Mol Med Rep. 2013;7(1):205–210.View this article via: PubMed Google Scholar
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241.
- Shouval DS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40(5):706–719.
- Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2014;14(1):51–59.
-
Version history
- Version 1 (September 1, 2015): No description
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Review Series
Cancer Immunotherapy
-
Therapeutic cancer vaccines
Cornelis J.M. Melief et al. -
From mice to humans: developments in cancer immunoediting
Michele W.L. Teng et al. -
Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected
Douglas Marvel et al. -
Tumor neoantigens: building a framework for personalized cancer immunotherapy
Matthew M. Gubin et al. -
Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages
Stefano Ugel et al. -
Immunity, inflammation, and cancer: an eternal fight between good and evil
Shabnam Shalapour et al. -
Cancer immunotherapy: harnessing the immune system to battle cancer
Yiping Yang -
CAR therapy: the CD19 paradigm
Michel Sadelain -
Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future
Lieping Chen et al. -
Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade
Elizabeth Buchbinder et al.
Metrics
Go to
- Top
- Abstract
- Introduction
- The evil: chronic inflammation and cancer
- The good: immunity and cancer
- Innate immune cells: the good APC and the evil TAM, TAN, and MDSC
- Janus-faced innate lymphocytes: NK, NKT, and γδ T cells
- CAFs: underappreciated immune regulators
- Dual roles of T cell subsets in cancer
- Good and bad B cells
- The molecular “Yin-Yang” of cancer inflammation and immunity
- Conclusions and prospects
- Acknowledgments
- Footnotes
- References
- Version history



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)


