Cancer Metabolism
Series edited by Matthew Vander Heiden
An association between cancer and altered cellular metabolism has been known for decades, and clinically this aspect of tumor biology is exploited by imaging techniques that rely on the uptake of labeled glucose analogues to identify tumors. Following the discovery of oncogenic mutations in several metabolic enzymes, there has been a resurgence of interest in recent years on the role that altered metabolism plays in tumor development and maintenance. The reviews in this series address many key facets of cancer metabolism, including the role of isocitrate dehydrogenase (IDH) mutations and oncometabolites, the link between tumor hypoxia and metabolism, as well as lactate and glucose metabolism in tumors. Further, these reviews collectively explore emerging therapeutic and imaging options that target metabolic pathways in cancer. Image credit, Drs. Changho Choi and Elizabeth Maher.
Articles in series
Abstract
The metabolism of cancer cells differs from most normal cells, but how to exploit this difference for patient benefit is incompletely understood. Cancer cells require altered metabolism to efficiently incorporate nutrients into biomass and support abnormal proliferation. In addition, the survival of tumor cells outside of a normal tissue context requires adaptation of metabolism to different microenvironments. Some existing chemotherapies target metabolic enzymes, and there is a resurgent interest in developing new cancer drugs that interfere with metabolism. Success with this approach depends on understanding why specific metabolic pathways are important for cancer cells, determining how best to select patients, and developing technologies for monitoring patient response to therapies that target metabolic enzymes. The articles in this Review series address these issues, with a focus on how altered metabolism might influence tumor progression and how this knowledge might inform the use of new therapies targeting cancer metabolism. Emerging biomarker strategies to guide drug development are also highlighted.
Authors
Matthew G. Vander Heiden
Abstract
The discovery of cancer-associated mutations in genes encoding key metabolic enzymes has provided a direct link between altered metabolism and cancer. Advances in mass spectrometry and nuclear magnetic resonance technologies have facilitated high-resolution metabolite profiling of cells and tumors and identified the accumulation of metabolites associated with specific gene defects. Here we review the potential roles of such “oncometabolites” in tumor evolution and as clinical biomarkers for the detection of cancers characterized by metabolic dysregulation.
Authors
Ming Yang, Tomoyoshi Soga, Patrick J. Pollard
Abstract
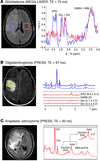
The investigation of metabolic pathways disturbed in isocitrate dehydrogenase (IDH) mutant tumors revealed that the hallmark metabolic alteration is the production of D-2-hydroxyglutarate (D-2HG). The biological impact of D-2HG strongly suggests that high levels of this metabolite may play a central role in propagating downstream the effects of mutant IDH, leading to malignant transformation of cells. Hence, D-2HG may be an ideal biomarker for both diagnosing and monitoring treatment response targeting IDH mutations. Magnetic resonance spectroscopy (MRS) is well suited to the task of noninvasive D-2HG detection, and there has been much interest in developing such methods. Here, we review recent efforts to translate methodology using MRS to reliably measure in vivo D-2HG into clinical research.
Authors
Ovidiu C. Andronesi, Otto Rapalino, Elizabeth Gerstner, Andrew Chi, Tracy T. Batchelor, Dan P. Cahill, A. Gregory Sorensen, Bruce R. Rosen
Abstract
Hypoxia occurs frequently in human cancers and induces adaptive changes in cell metabolism that include a switch from oxidative phosphorylation to glycolysis, increased glycogen synthesis, and a switch from glucose to glutamine as the major substrate for fatty acid synthesis. This broad metabolic reprogramming is coordinated at the transcriptional level by HIF-1, which functions as a master regulator to balance oxygen supply and demand. HIF-1 is also activated in cancer cells by tumor suppressor (e.g., VHL) loss of function and oncogene gain of function (leading to PI3K/AKT/mTOR activity) and mediates metabolic alterations that drive cancer progression and resistance to therapy. Inhibitors of HIF-1 or metabolic enzymes may impair the metabolic flexibility of cancer cells and make them more sensitive to anticancer drugs.
Authors
Gregg L. Semenza
Abstract
Recent genome-wide discovery studies have identified a spectrum of mutations in different malignancies and have led to the elucidation of novel pathways that contribute to oncogenic transformation. The discovery of mutations in the genes encoding isocitrate dehydrogenase (IDH) has uncovered a critical role for altered metabolism in oncogenesis, and the neomorphic, oncogenic function of IDH mutations affects several epigenetic and gene regulatory pathways. Here we discuss the relevance of IDH mutations to leukemia pathogenesis, therapy, and outcome and how mutations in IDH1 and IDH2 affect the leukemia epigenome, hematopoietic differentiation, and clinical outcome.
Authors
Anna Sophia McKenney, Ross L. Levine
Abstract
Glutamine is an abundant and versatile nutrient that participates in energy formation, redox homeostasis, macromolecular synthesis, and signaling in cancer cells. These characteristics make glutamine metabolism an appealing target for new clinical strategies to detect, monitor, and treat cancer. Here we review the metabolic functions of glutamine as a super nutrient and the surprising roles of glutamine in supporting the biological hallmarks of malignancy. We also review recent efforts in imaging and therapeutics to exploit tumor cell glutamine dependence, discuss some of the challenges in this arena, and suggest a disease-focused paradigm to deploy these emerging approaches.
Authors
Christopher T. Hensley, Ajla T. Wasti, Ralph J. DeBerardinis
Abstract
Lactate, once considered a waste product of glycolysis, has emerged as a critical regulator of cancer development, maintenance, and metastasis. Indeed, tumor lactate levels correlate with increased metastasis, tumor recurrence, and poor outcome. Lactate mediates cancer cell intrinsic effects on metabolism and has additional non–tumor cell autonomous effects that drive tumorigenesis. Tumor cells can metabolize lactate as an energy source and shuttle lactate to neighboring cancer cells, adjacent stroma, and vascular endothelial cells, which induces metabolic reprogramming. Lactate also plays roles in promoting tumor inflammation and in functioning as a signaling molecule that stimulates tumor angiogenesis. Here we review the mechanisms of lactate production and transport and highlight emerging evidence indicating that targeting lactate metabolism is a promising approach for cancer therapeutics.
Authors
Joanne R. Doherty, John L. Cleveland
Abstract
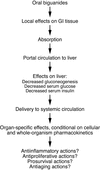
Metformin is widely prescribed for the treatment of type II diabetes. Recently, it has been proposed that this compound or related biguanides may have antineoplastic activity. Biguanides may exploit specific metabolic vulnerabilities of transformed cells by acting on them directly, or may act by indirect mechanisms that involve alterations of the host environment. Preclinical data suggest that drug exposure levels are a key determinant of proposed direct actions. With respect to indirect mechanisms, it will be important to determine whether recently demonstrated metformin-induced changes in levels of candidate systemic mediators such as insulin or inflammatory cytokines are of sufficient magnitude to achieve therapeutic benefit. Results of the first generation of clinical trials now in progress are eagerly anticipated. Ongoing investigations may justify a second generation of trials that explore pharmacokinetic optimization, rational drug combinations, synthetic lethality strategies, novel biguanides, and the use of predictive biomarkers.
Authors
Michael Pollak



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)