Abstract
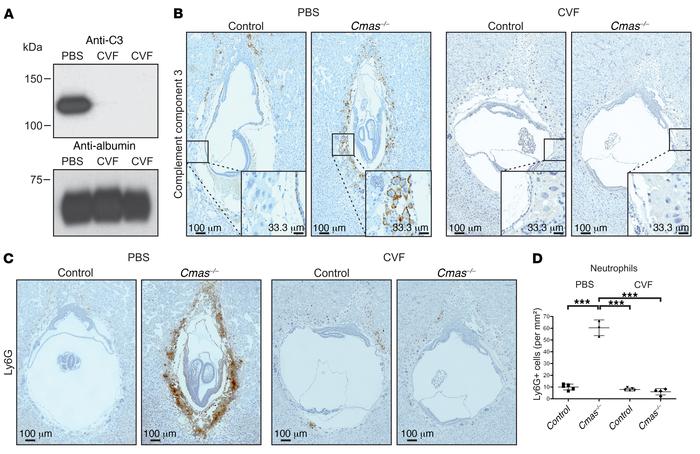
The negatively charged sugar sialic acid (Sia) occupies the outermost position in the bulk of cell surface glycans. Lack of sialylated glycans due to genetic ablation of the Sia-activating enzyme CMP–sialic acid synthase (CMAS) resulted in embryonic lethality around day 9.5 post coitum (E9.5) in mice. Developmental failure was caused by complement activation on trophoblasts in Cmas–/– implants and was accompanied by infiltration of maternal neutrophils at the fetal-maternal interface, intrauterine growth restriction, impaired placental development, and a thickened Reichert’s membrane. This phenotype, which shared features with complement receptor 1-related protein Y (Crry) depletion, was rescued in E8.5 Cmas–/– mice upon injection of cobra venom factor, resulting in exhaustion of the maternal complement component C3. Here we show that Sia is dispensable for early development of the embryo proper but pivotal for fetal-maternal immune homeostasis during pregnancy, i.e., for protecting the allograft implant against attack by the maternal innate immune system. Finally, embryos devoid of cell surface sialylation suffered from malnutrition due to inadequate placentation as a secondary effect.
Authors
Markus Abeln, Iris Albers, Ulrike Peters-Bernard, Kerstin Flächsig-Schulz, Elina Kats, Andreas Kispert, Stephen Tomlinson, Rita Gerardy-Schahn, Anja Münster-Kühnel, Birgit Weinhold
Figure 6
CVF decomplements the maternal serum and rescues the inflammatory phenotype of



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)