Advertisement
CorrigendumNeuroscience Free access | 10.1172/JCI36246C1
Immune cell–derived opioids protect against neuropathic pain in mice
Dominika Labuz, Yvonne Schmidt, Anja Schreiter, Heike L. Rittner, Shaaban A. Mousa, and Halina Machelska
Find articles by Labuz, D. in: PubMed | Google Scholar
Find articles by Schmidt, Y. in: PubMed | Google Scholar
Find articles by Schreiter, A. in: PubMed | Google Scholar
Find articles by Rittner, H. in: PubMed | Google Scholar
Find articles by Mousa, S. in: PubMed | Google Scholar
Find articles by Machelska, H. in: PubMed | Google Scholar
Published April 1, 2009 - More info
J Clin Invest. 2009;119(4):1051–1051. https://doi.org/10.1172/JCI36246C1.
© 2009 The American Society for Clinical Investigation
Related article:
Abstract
The analgesic effects of leukocyte-derived opioids have been exclusively demonstrated for somatic inflammatory pain, for example, the pain associated with surgery and arthritis. Neuropathic pain results from injury to nerves, is often resistant to current treatments, and can seriously impair a patient’s quality of life. Although it has been recognized that neuronal damage can involve inflammation, it is generally assumed that immune cells act predominately as generators of neuropathic pain. However, in this study we have demonstrated that leukocytes containing opioids are essential regulators of pain in a mouse model of neuropathy. About 30%–40% of immune cells that accumulated at injured nerves expressed opioid peptides such as β-endorphin, Met-enkephalin, and dynorphin A. Selective stimulation of these cells by local application of corticotropin-releasing factor led to opioid peptide–mediated activation of opioid receptors in damaged nerves. This ultimately abolished tactile allodynia, a highly debilitating heightened response to normally innocuous mechanical stimuli, which is symptomatic of neuropathy. Our findings suggest that selective targeting of opioid-containing immune cells promotes endogenous pain control and offers novel opportunities for management of painful neuropathies.
Authors
Dominika Labuz, Yvonne Schmidt, Anja Schreiter, Heike L. Rittner, Shaaban A. Mousa, Halina Machelska
Original citation: J. Clin. Invest.119:278–286 (2009). doi:10.1172/JCI36246.
Citation for this corrigendum: J. Clin. Invest.119:1051 (2009). doi:10.1172/JCI36246C1.
During the preparation of the manuscript, the END and ENK images on the left side of Figure 1C were inadvertently transposed. The correct Figure 1C appears below.
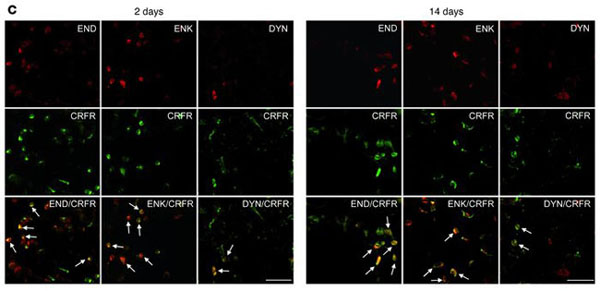
In the Discussion, the final sentence of the first paragraph should read:
Our current study offers a possible advancement in neuropathic pain therapy based on the utilization of the physiological functions of leukocyte-derived opioids that represents control of painful neuropathies without CNS side effects.
In the legend to Figure 5, the penultimate sentence should read:
Right panels at 2 days and 14 days following CCI: Injection (s.c.) at the neck of the most effective near-nerve doses of CTOP (0.25 µg), ICI 174,864 (2 µg), norBNI (10 µg), and NLXM (5 µg) did not reverse near-nerve CRF-induced (20 ng at 2 days or 100 ng at 14 days) analgesia (P > 0.05; ANOVA).
The authors regret the errors.
-
Version history
- Version 1 (April 1, 2009): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)
