Citation Information: J Clin Invest. 2019;129(10):4506-4522. https://doi.org/10.1172/JCI128503.
Abstract
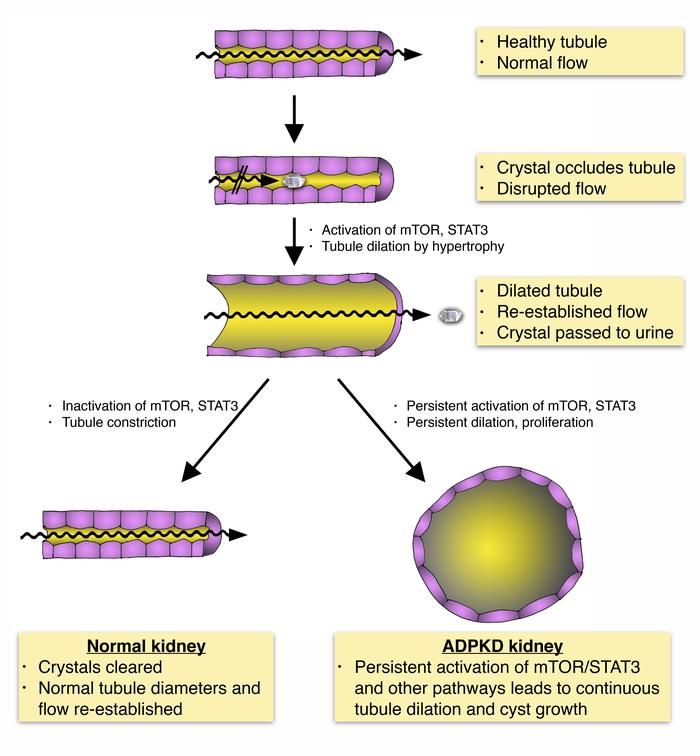
The rate of disease progression in autosomal-dominant polycystic kidney disease (ADPKD) has high intrafamilial variability, suggesting that environmental factors may play a role. We hypothesized that a prevalent form of renal insult may accelerate cystic progression and investigated tubular crystal deposition. We report that calcium oxalate (CaOx) crystal deposition led to rapid tubule dilation, activation of PKD-associated signaling pathways, and hypertrophy in tubule segments along the affected nephrons. Blocking mTOR signaling blunted this response and inhibited efficient excretion of lodged crystals. This mechanism of “flushing out” crystals by purposefully dilating renal tubules has not to our knowledge been previously recognized. Challenging PKD rat models with CaOx crystal deposition or inducing calcium phosphate deposition by increasing dietary phosphorus intake led to increased cystogenesis and disease progression. In a cohort of patients with ADPKD, lower levels of urinary excretion of citrate, an endogenous inhibitor of calcium crystal formation, were correlated with increased disease severity. These results suggest that PKD progression may be accelerated by commonly occurring renal crystal deposition that could be therapeutically controlled by relatively simple measures.
Authors
Jacob A. Torres, Mina Rezaei, Caroline Broderick, Louis Lin, Xiaofang Wang, Bernd Hoppe, Benjamin D. Cowley Jr., Vincenzo Savica, Vicente E. Torres, Saeed Khan, Ross P. Holmes, Michal Mrug, Thomas Weimbs
Graphical abstract



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)