Seeing red in the liver
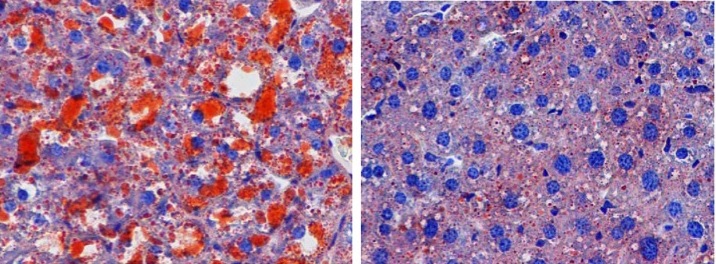
Patients with non-alcoholic fatty liver disease (NAFLD) have an abnormal accumulation of fat within hepatocytes. While most individuals with NAFLD have no symptoms or complications, in some patients, the accumulation of fatty acids promotes inflammation and can lead to hepatic fibrosis and scarring. NAFLD is strongly associated with obesity; however, the cellular mechanisms that induce hepatic fat accumulation are not fully understood. Yan Lu, Xing Liu, Yang Jiao, and colleagues at the Shanghai Jiao Tong University School of Medicine found that the secreted cellular adhesion protein periostin is substantially upregulated in the livers of obese humans and mice. In WT mice, overexpression of periostin promoted triglyceride accumulation in the liver and serum. Conversely, reduction of periostin improved hepatosteatosis in obese animals. The authors determined that periostin binds α6β4 integrin, resulting in JNK-mediated repression of PPARα. Inhibition of PPARα impaired fatty acid oxidation and promoted triglyceride accumulation. These studies identify a previously unappreciated role for periostin in lipid metabolism and fatty liver disease, suggesting that it could serve as a therapeutic target in diseases characterized by dyslipidemia. In the accompanying image, hepatic sections from WT mice (left) or periostin-deficient mice (right) fed a high-fat diet were stained with Oil Red O. Loss of periostin prevents triglyceride (red) accumulation in the liver.
Related articles
Citation Information: J Clin Invest. 2014;124(8):3501-3513. https://doi.org/10.1172/JCI74438.
Abstract
Hepatosteatosis is characterized by an aberrant accumulation of triglycerides in the liver; however, the factors that drive obesity-induced fatty liver remain largely unknown. Here, we demonstrated that the secreted cell adhesion protein periostin is markedly upregulated in livers of obese rodents and humans. Notably, overexpression of periostin in the livers of WT mice promoted hepatic steatosis and hypertriglyceridemia. Conversely, both genetic ablation of periostin and administration of a periostin-neutralizing antibody dramatically improved hepatosteatosis and hypertriglyceridemia in obese mice. Overexpression of periostin resulted in reduced expression of peroxisome proliferator–activated receptor α (PPARα), a master regulator of fatty acid oxidation, and activation of the JNK signaling pathway. In mouse primary hepatocytes, inhibition of α6β4 integrin prevented activation of JNK and suppression of PPARα in response to periostin. Periostin-dependent activation of JNK resulted in activation of c-Jun, which prevented RORα binding and transactional activation at the
Authors
Yan Lu, Xing Liu, Yang Jiao, Xuelian Xiong, E Wang, Xiaolin Wang, Zhijian Zhang, Huijie Zhang, Lingling Pan, Youfei Guan, Dongsheng Cai, Guang Ning, Xiaoying Li



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

