Citations to this article
Abstract
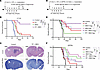
Radiation therapy (RT) is the standard of care for glioblastoma but is not curative. Triggering the cGAS/stimulator of interferon genes (STING) pathway with potent agonists, such as 8803, exerts activity across high-grade glioma preclinical models. To determine if the combination of 8803 with RT warrants consideration in the up-front treatment setting and to clarify the underlying mechanisms of therapeutic activity, C57BL/6J mice harboring intracerebral CT-2A or QPP8v gliomas were treated with RT, intratumoral 8803, or both. The treatment with the combination resulted in 80% long-term survival in the CT-2A model but not in the radiation-resistant QPP8v model. This therapeutic effect was maintained in Sting–/– CT-2A cells, highlighting the direct role of the immune system in mediating the survival benefit. Single-cell RNA-Seq identified increased nitric oxide synthase 2 (Nos2) in inflammatory tumor-associated macrophages; however, the therapeutic effect was maintained in Nos2–/– mice. Additionally, 8803 reprogrammed the blood-brain barrier (BBB) by altering the Pecam and Cd147 pathways in endothelial cells; intracranial injection of 8803 induced bihemispheric BBB opening for up to 24 hours. Sting activation was visualized longitudinally using 3’-deoxy-3’-[18F]-fluorothymidine ([18F]-FLT) PET, which peaked 72–96 hours after 8803 administration. In summary, 8803 combined with RT triggers distinctive antiglioma immune reactivity, facilitates BBB opening, and warrants consideration for up-front clinical trials in glioblastoma, where treatment effects can be monitored using [18F]-FLT PET imaging.
Authors
Shashwat Tripathi, Hinda Najem, Lisa Hurley, Ruochen Du, Crismita Dmello, Heba Ali, Kathleen McCortney, Karl J. Habashy, Peng Zhang, Craig M. Horbinski, Lara Leoni, Ryan J. Avery, Rimas V. Lukas, Timothy L. Sita, David R. Raleigh, Sean Sachdev, Roger Stupp, Maciej S. Lesniak, David M. Ashley, Daniele Procissi, Michael A. Curran, Irina Balyasnikova, Amy B. Heimberger
Loading citation information...




Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)