Advertisement
Review
Open Access |  10.1172/JCI196348
10.1172/JCI196348
Citrullination in tumor immunity and therapy
Michael R. Pitter1,2 and Weiping Zou1,2,3,4,5,6
1Department of Surgery, University of Michigan Medical School, Ann Arbor, Michigan, USA.
2Center of Excellence for Cancer Immunology and Immunotherapy, University of Michigan Rogel Cancer Center, Ann Arbor, Michigan, USA.
3Graduate Program in Immunology, University of Michigan, Ann Arbor, Michigan, USA.
4Department of Pathology, University of Michigan Medical School, Ann Arbor, Michigan, USA.
5Graduate Program in Cancer Biology and
6University of Michigan Rogel Cancer Center, University of Michigan, Ann Arbor, Michigan, USA.
Address correspondence to: Weiping Zou, Departments of Pathology and Surgery, University of Michigan School of Medicine, 109 Zina Pitcher Place, Ann Arbor Michigan 48109-0669, USA. Email: wzou@umich.edu.
Find articles by Pitter, M. in: PubMed | Google Scholar
1Department of Surgery, University of Michigan Medical School, Ann Arbor, Michigan, USA.
2Center of Excellence for Cancer Immunology and Immunotherapy, University of Michigan Rogel Cancer Center, Ann Arbor, Michigan, USA.
3Graduate Program in Immunology, University of Michigan, Ann Arbor, Michigan, USA.
4Department of Pathology, University of Michigan Medical School, Ann Arbor, Michigan, USA.
5Graduate Program in Cancer Biology and
6University of Michigan Rogel Cancer Center, University of Michigan, Ann Arbor, Michigan, USA.
Address correspondence to: Weiping Zou, Departments of Pathology and Surgery, University of Michigan School of Medicine, 109 Zina Pitcher Place, Ann Arbor Michigan 48109-0669, USA. Email: wzou@umich.edu.
Find articles by Zou, W. in: PubMed | Google Scholar
Published October 15, 2025 - More info
J Clin Invest. 2025;135(20):e196348. https://doi.org/10.1172/JCI196348.
© 2025 Pitter, et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Peptidyl arginine deiminases (PADs) catalyze the conversion of arginine residues into peptidyl citrulline, a posttranslational modification known as protein citrullination (or arginine deimination). This process alters the charge of proteins from positive to neutral, thereby affecting their folding, stability, conformation, and function. PAD2 and PAD4 can translocate into the nucleus and citrullinate both cytoplasmic and nuclear proteins. In this Review, we focus on PAD2- and PAD4-mediated citrullination in immune cell subsets within the tumor microenvironment. We discuss how citrullination regulates immune cell function and tumor immunity and explore the potential of targeting citrullination as a strategy for cancer immunotherapy.
-
Introduction
The genome gives rise to the transcriptome, which, in turn, gives rise to the proteome (1, 2). Posttranslational modifications (PTMs) shape final protein functions (3–8). PTMs influence protein folding, stability, and three-dimensional conformation (9–11). These modifications include the covalent addition of chemical groups (such as phosphorylation, methylation, acylation, and acetylation); the addition of large unique molecules (such as ADP-ribosylation, prenylation, and glycosylation); the addition of polypeptides (such as ubiquitination and SUMOylation); proteolytic cleavage; and the modification of amino acids (such as glycation, oxidation, carbamylation, and citrullination) (12, 13). One key distinction among PTMs is their reversibility (12, 13). Most PTMs are reversible, while others — such as proteolysis and citrullination — are irreversible (3, 12, 14–17). Proteolysis, though common, often results in protein inactivation (18, 19), whereas citrullination is the only known irreversible enzymatic PTM that modifies amino acid identity by converting arginine into citrulline (13, 20). The discovery of citrullinated proteins dates to 1958, when G.E. Rogers and colleagues reported the presence of citrulline in fibrous proteins of rat hair follicles (21), building on earlier observations in red algae (22). The identification of citrulline in human brain myelin proteins in 1971 marked a turning point in citrullination research (23, 24). Citrullination involves peptidyl arginine deiminase–catalyzed (PAD-catalyzed) hydrolysis of the guanidino group in peptidyl arginine, converting it into urea and releasing ammonia, thereby forming peptidyl citrulline (25, 26) (Figure 1). This modification alters a protein’s structure and function (3, 27–31). Later studies revealed that PAD2 and PAD4 citrullinate histones, affecting chromatin structure and gene transcription (32–36). Histone citrullination can promote neutrophil extracellular trap (NET) formation, DNA damage responses, and transcriptional regulation (32, 34). However, the roles of PAD2 and PAD4 in nonhistone protein citrullination within the tumor microenvironment (TME) remain largely unexplored. This Review highlights citrullination in immune cells and its implications for tumor immunity and therapy.
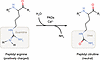 Figure 1
Figure 1Scheme of PAD-mediated citrullination. PADs catalyze the conversion of arginine residues into peptidyl citrulline. This process alters the charge of proteins from positive to neutral, thereby affecting protein folding, stability, conformation, and thereby function.
-
PAD family member expression and distribution
The PAD family of isozymes — PAD1, PAD2, PAD3, PAD4, and PAD6 — catalyzes protein citrullination (25, 26) (Figure 1). PAD1 is predominantly expressed in the epidermis, hair follicles, uterus, and stomach; PAD3 in hair follicles, skin, and peripheral nerves; and PAD6 in reproductive tissues, including ovaries, oocytes, embryos, and testes (25, 37). Notably, PAD6 lacks several conserved Ca²+-binding residues required for enzymatic activity (38), and its catalytic function has not been demonstrated (39, 40).
PAD2 exhibits broad tissue distribution, including skeletal muscle, salivary glands, brain, skin, peripheral nerves, uterus, pancreas, kidney, and inner ear. PAD4 is more restricted, primarily expressed in the brain and uterus. Importantly, PAD2 and PAD4 are the major PAD isozymes found in immune cells (25, 35).
PAD4 uniquely possesses a canonical nuclear localization signal (NLS), enabling its translocation to the nucleus, where it citrullinates histones — an essential step in NET formation in neutrophils (32, 33, 41). Although PAD2 lacks a canonical NLS, it can also localize to nucleus and modify nuclear substrates (35, 42, 43). Histone citrullination by PADs can influence chromatin architecture and epigenetically regulate transcription (33, 34, 44).
The functional consequences of PAD-mediated citrullination are determined by their targeted substrates. PAD2 and PAD4 target various cytoplasmic and nuclear proteins, including transcription factors and coregulators (45, 46) (Figure 2A). Their expression in hematopoietic progenitors and immune cells and their ability to enter the nucleus suggest important roles in modulating immune cell differentiation, phenotype, and function (Figure 2B) (47).
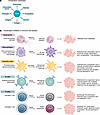 Figure 2
Figure 2PAD-mediated citrullination. (A) Citrullination substrates. (B) Citrullination inhibition in immune cell populations and potential biological outcomes.
-
Citrullination in immune cells and tumor immunity
Given that citrullination occurs in immune cells, it is expected to influence tumor immune responses. Here, we review both the established and emerging roles of PAD-mediated citrullination across various immune cell subsets within the TME.
Citrullination in neutrophils. Most studies of PADs in neutrophils have focused on autoimmune conditions. Inflammatory stimuli, including ROS, TLR agonists, and proinflammatory cytokines can drive intracellular Ca²+ influx and activate PAD4, leading to histone citrullination, chromatin decondensation, and NET formation (48–51). Additionally, PAD4 citrullinates p65 in response to LPS stimulation, thereby inducing transcription of inflammatory cytokines, such as IL-1β and TNF-α (52). Cancer cells can exploit NETs to promote metastasis (53, 54). Chemotherapy-induced NET formation also contributes to immune evasion and resistance via TGF-β signaling (55–58). NETs can reactivate dormant tumor cells and promote metastatic outgrowth (59). Inhibiting PAD4 or degrading chromatin with DNase I have been shown to reduce tumor metastasis and prolong survival in tumor-bearing models (60). PAD2 also contributes to NET formation by citrullinating histones (61). Thus, PADs in neutrophils may promote a proinflammatory milieu, facilitating tumor progression.
Notably, there may exist a distinct antitumorigenic neutrophil subset, positively correlating with immune checkpoint blockade (ICB) efficacy (62). In line with this, NETs induced by chemotherapy have been shown to release tumor-killing granules, containing elastase, myeloperoxidase, and cathepsin G, in a murine colon cancer model (63). It remains to be studied how PADs affect different neutrophil subsets and ICB in patients with cancer.
Citrullination in macrophages. Tumor-associated macrophages (TAMs) can play an immunosuppressive role in antitumor immune responses via distinct mechanisms (64). For example, antigen-presenting cells, including macrophages and DCs, express high levels of PD-L1 (also known as B7-H1) in the human TME and tumor-draining lymph nodes, contributing to immune resistance to ICB in the TME (65–68). Interestingly, PAD4 is highly expressed in macrophages (47, 69), including TAMs (70). It is not clear if PD-L1 and PAD4 are differentially expressed in TAMs. PAD4 citrullinates STAT1 in TAMs, enhancing the interaction between STAT1 and protein inhibitor of activated STAT1 (PIAS1) and suppressing STAT1 transcriptional activity (70). This attenuates class II transactivator (CIITA) transcription and reduces MHC-II expression in TAMs, thereby impairing antigen presentation and T cell activation (70–72) (Figure 3). PAD2 and PAD4 also regulate inflammasome activation and pyroptosis in macrophages. For example, PAD2 citrullinates apoptosis-associated speck-like protein containing a CARD (ASC) specks, leading to caspase-1 activation and IL-1β and IL-18 release (73, 74). Inhibition of PAD4 with the small-molecule inhibitor GSK484 reduces IL-1β secretion following NOD-like receptor family pyrin domain containing 3 (NLRP3) activation. PAD2-specific inhibition with AFM30a more effectively diminishes IL-1β production in PAD4-deficient macrophages, suggesting functional redundancy and synergy of PAD2 and PAD4 (73). PAD2 knockout reduces pyroptosis in bone marrow–derived macrophages and alveolar macrophages (74). Thus, PAD2 and PAD4 generally support inflammatory cytokine production in macrophages.
 Figure 3
Figure 3The relationship between PAD4 and MHC-II expression in macrophages. PAD4 mediates citrullination of STAT1 in tumor-associated macrophages (TAMs). This results in enhanced interaction between STAT1 and PIAS1, thereby reducing STAT1 binding to the CIITA gene promoter, downregulating MHC-II expression, and consequently impairing tumor immunity. The opposite phenotype is shown in PAD4–/– (PAD4-deficient) macrophages.
Moreover, like neutrophils, macrophages can also form extracellular traps (METs). PAD2 and PAD4 promote MET formation in autoimmune models (75, 76). METs can exacerbate or alleviate tumor progression depending on the context (77–81). While inflammatory cytokines can target tumor cells and stromal cells to promote tumor growth, they can also stimulate antigen-presenting cells, enhancing their antigen presentation and T cell priming and activation. Thus, PADs in macrophages may play dual roles in tumor immunity depending on their cellular targets and immune context in the TME. Given that treatment with PAD4 inhibitors (GSK484 and JBI-589) reduces tumor progression and enhances therapeutic efficacy of ICB in different tumor-bearing mouse models (70, 82), inhibition of PAD4 is a potential strategy for cancer immunotherapy.
Citrullination in T cells. Citrullination is relatively understudied in T cells. A seminal study showed that PAD2 can citrullinate the transcription factors GATA-binding protein 3 (GATA-3) and retinoic acid–related orphan receptor γ, thymus-specific isoform (RORγt), thereby modulating Th cell polarization. Specifically, PAD2-mediated citrullination of GATA-3 reduced IL-13 promoter activity, whereas citrullination of RORγt enhanced IL-17A promoter activity, thereby promoting Th17 differentiation (83). Structural analysis revealed that citrullination of arginine 330 (R330) on GATA-3 — a residue that interacts with the DNA backbone — disrupted its DNA binding and dampened Th2 polarization. In contrast, citrullination of RORγt enhanced its DNA binding and transcriptional activity, favoring Th17 lineage commitment (83). Although this study was conducted in an inflammatory setting, it suggests that PAD2 may influence CD4+ T cell polarization within the TME. Another study demonstrated that PAD2 and/or PAD4 deficiency impairs both Th1 and Th17 cell differentiation in a TLR7-driven lupus model (84). Given that Th1 and Th17 cells play important roles in antitumor immunity (85–88), these findings raise the possibility that PADs modulate effector T cell responses in the TME.
Moreover, PAD2 can citrullinate chemokines such as CXCL10 and CXCL11 — key mediators of T cell trafficking — thereby impairing T cell migration to inflammatory sites, including tumors (89, 90). Although this mechanism has not yet been explored in the context of cancer, altered chemokine citrullination could influence effector T cell trafficking into the TME.
In autoimmune settings, such as rheumatoid arthritis (RA), both CD4+ and CD8+ T cells recognize citrullinated self-antigens, including vimentin, fibrinogen, and enolase (91–93). Analogously, whether T cell responses are triggered by citrullinated tumor or stromal proteins in cancer remains unknown. Altogether, these findings suggest that citrullination may shape T cell phenotype, tumor migration, and the antigenic landscape of tumors, thereby impacting T cell–mediated tumor immunity.
Citrullination in other immune cells. In addition to neutrophils, macrophages, and T cells, citrullination has also been studied in B cells and DCs (94). PAD2 citrullinates marginal zone B and B1 cell–specific protein (MZB1) at multiple sites, modulating B cell activation, as well as antibody production and secretion (95). Pharmacological inhibition of PAD2 with AFM30a significantly reduced IgA and IgM levels in human plasmablasts, while IgG levels remained unaffected (95). Like T cells in RA, autoreactive B cells can respond to citrullinated proteins — such as collagen type II — in the synovial fluid, further exacerbating inflammation in patients with RA (92).
In DCs, PAD inhibition alters cytokine production and functional maturation. For instance, the pan-PAD inhibitor Cl-amidine reduces DC secretion of TNF-α, IL-6, IL-1β, and IL-12p70 in response to various TLR agonists (96). Consistent with this, Cl-amidine also downregulates inducible nitric oxide synthase (iNOS) expression in DCs (97). Notably, iNOS deficiency has been associated with enhanced tumor progression in melanoma models (98, 99). PADs also regulate the transdifferentiation of DCs into osteoclasts. Krishnamurthy et al. found that PAD2 and PAD4 are expressed during this process — with PAD2 predominantly in the cytoplasm and PAD4 in the nucleus — and that multiple substrates, including actin and vimentin, are citrullinated before, during, and after this transition (100). Inhibition with Cl-amidine significantly impaired osteoclast differentiation from immature DCs. Given that tumors, such as prostate and breast cancer, frequently metastasize to the bone marrow, it will be important to investigate whether PAD activity contributes to or modulates tumor bone marrow metastasis. Together, these findings suggest that PAD2 and PAD4 regulate B cell and DC biology in both physiological and pathological conditions, with potential implications for shaping tumor immune responses and therapeutic outcomes.
-
Potential relationship between arginine methylation and citrullination
Arginine methylation and citrullination often act as functionally antagonistic modifications (101, 102). For example, STAT1 arginine methylation enhances its transcriptional activity in IFN-stimulated U266B1, 2fTGH, and HEK 293T cell lines (103), whereas PAD4-mediated citrullination of STAT1 promotes its association with PIAS1, thereby reducing STAT1 binding to CIITA promoter regions and impairing MHC-II expression in macrophages (70).
Emerging evidence suggests that arginine methylation is essential for antitumor immunity. For example, protein arginine methyltransferase 5 (PRMT5) can mediate methylation of Sm proteins in T cells. This supports γc-family cytokine signaling by facilitating the splicing of Il2rg and Jak3 transcripts, leading to proper expression of IL-2 receptor and Janus kinase 3 (JAK3) (104). This observation raises the possibility that PAD-mediated citrullination could antagonize γc-cytokine signaling, thereby impairing T cell responses to tumors. Similarly, methylation of the nuclear factor of activated T cells (NF-AT) coactivator NF-AT–interacting protein 45 (NIP45, also known as ILF2BP) is required for IFN-γ production in T cells, and its inhibition reduces T cell cytokine expression (105). Thus, citrullination and methylation may compete for the same arginine residues on immune regulatory proteins, shaping immune cell function in the TME (106–111). Comprehensive proteomic profiling of methylated and citrullinated proteins in immune cells will help identify novel PAD substrates and elucidate the interplay between these competing PTMs in modulating tumor immunity (26, 40, 112).
-
Therapeutically targeting citrullination
PAD inhibitors were initially developed for treating autoimmune diseases such as RA (113–117). More recently, these inhibitors have been applied to target citrullination in cancer cells and tumor-infiltrating neutrophils (118) (Table 1).
PAD inhibitors include both pan-PAD inhibitors and those selective for individual isozymes. Activity-based protein profiling high-throughput screening has identified reversible and irreversible inhibitors. Reversible inhibitors such as minocycline, chlortetracycline, ruthenium red, and sanguinarine typically act by chelating calcium, which is essential for PAD activation (119–122). Irreversible PAD inhibitors can covalently bind to the active-site cysteine of PADs, directly inhibiting enzymatic activity (114, 123, 124). Other irreversible PAD inhibitors such as NSC95397 and streptonigrin possess α,β-unsaturated carbonyl groups that covalently modify active-site cysteines (114). Streptonigrin shows high PAD4 selectivity and potency due to its substituted pyridyl and phenyl rings (125). GSK121 is a selective PAD4 inhibitor, and its further chemical optimization yielded GSK199 and GSK484, which exhibit enhanced potency and selectivity (126). 2-chloroacetamidine derivatives broadly inhibit PADs but show higher potency against PAD2 and PAD4. Cl-amidine, BB-Cl-amidine, and F-amidine are such derivatives; F-amidine and GSK484 are PAD4 selective (126–130). YW3-56, a Cl-amidine derivative, inhibits PAD1-4 with improved potency (131, 132). BMS-P5 and JBI-589 are next-generation PAD4 inhibitors that suppress NET formation and neutrophil recruitment, mitigating tumor progression in preclinical models (82, 133). Notably, BB-Cl-amidine may also act as a STING inhibitor and impair innate tumor immune responses (134). Additionally, GSK484 and JBI-589 can inhibit PAD4 by binding the calcium-free form of the isozyme and, therefore, may potentially be disengaged by high levels of calcium, particularly in the extracellular microenvironment (135, 136).
PAD2-selective inhibitors include benzimidazole-based compounds such as AFM30a, AFM32a, AFM41a, and AFM49a. These compounds have shown efficacy in preclinical models of PAD2-driven sepsis, endotoxic shock, and thrombosis (137–140). Recent studies using phage display have identified monoclonal antibodies that selectively activate or inhibit extracellular PAD4 activity, offering additional therapeutic avenues (141).
Overall, PAD inhibitors represent a promising class of therapeutic agents with potential application in cancer (Table 1). By disrupting PAD-mediated posttranslational and epigenetic modifications, these agents may modulate immune cell function and suppress tumor-promoting inflammation.
-
Conclusion
PTMs, including citrullination, are fundamental in regulating the structure, stability, and function of proteins. Citrullination modulates transcriptional programs in cancer cells and immune cells by altering protein conformation and protein-protein interactions (35, 142). In neutrophils, PAD4-mediated histone citrullination facilitates NET formation, which can enhance tumor progression and metastasis (54, 55). In macrophages, PAD-dependent citrullination regulates antigen presentation and inflammatory cytokine production (70). Despite these insights, the roles of PAD2 and PAD4 in other immune subsets, including T cells, B cells, and DCs, remain understudied. Notably, PAD4 directly citrullinates and activates NF-κB in neutrophils (52), indicating that there is an opportunity to explore the roles of NF-κB citrullination in other innate and adaptive immune cells whose effector functions are governed by NF-κB signaling.
Recent preclinical studies demonstrate that PAD inhibition can suppress tumor growth and metastasis, indicating a therapeutic potential in oncology (70, 82, 133). These findings underscore the need for a deeper mechanistic understanding of PAD isozyme activity within specific immune cell populations in the TME. Development of isozyme-selective inhibitors and novel PAD-targeting strategies could offer new immunotherapeutic avenues. Nevertheless, since the inhibition of PAD2 and PAD4 in tumor cells has shown antitumor efficacy in animal models (70, 82), targeting PAD activity in both immune and tumor cells may produce synergistic anticancer effects. For instance, PAD4 inhibition in TAMs enhances antigen presentation and T cell activation, and a similar mechanism in tumor cells could further promote tumor-specific T cell responses. Future research should focus on identifying citrullinated substrates in immune cells, dissecting PAD-mediated regulatory pathways, and evaluating PAD-targeted therapies in combination with different therapies, including ICB. By advancing our understanding of citrullination in cancer immunity, we may uncover novel opportunities for enhancing the efficacy of cancer immunotherapy.
-
Acknowledgments
We thank our laboratory members for their intellectual input. This work was supported, in part, by US NIH/National Cancer Institute (NIH/NCI) R01 research grants (CA217648, CA123088, CA099985, CA193136, and CA152470) and the NIH/NCI through the University of Michigan Rogel Cancer Center Grant (CA46592). Through acceptance of this federal funding, the NIH has been given a right to make the work publicly available in PubMed Central.
Address correspondence to: Weiping Zou, Departments of Pathology and Surgery, University of Michigan School of Medicine, 109 Zina Pitcher Place, Ann Arbor Michigan 48109-0669, USA. Email: wzou@umich.edu.
-
Footnotes
Conflict of interest: WZ has served as a scientific advisor or consultant for CStone, ProteoVant, Hanchor Bio, and NextCure.
Copyright: © 2025, Pitter et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(20):e196348. https://doi.org/10.1172/JCI196348.
-
References
- Crick F. Central dogma of molecular biology. Nature. 1970;227(5258):561–563.
- Thieffry D, Sarkar S. Forty years under the central dogma. Trends Biochem Sci. 1998;23(8):312–316.
- Liu J, et al. Unconventional protein post-translational modifications: the helmsmen in breast cancer. Cell Biosci. 2022;12(1):22.
- Ramazi S, Zahiri J. Posttranslational modifications in proteins: resources, tools and prediction methods. Database (oxford). 2021;2021:baab012.
- Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nat Biotechnol. 2003;21(3):255–261.
- Ryšlavá H, et al. Effect of posttranslational modifications on enzyme function and assembly. J Proteomics. 2013;92:80–109.
- Ahearn IM, et al. Regulating the regulator: post-translational modification of RAS. Nat Rev Mol Cell Biol. 2012;13(1):39–51.
- Liu J, et al. Post-translational modification control of innate immunity. Immunity. 2016;45(1):15–30.
- Duan G, Walther D. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput Biol. 2015;11(2):e1004049.
- Conibear AC. Deciphering protein post-translational modifications using chemical biology tools. Nat Rev Chem. 2020;4(12):674–695.
- Grotenbreg G, Ploegh H. Chemical biology: dressed-up proteins. Nature. 2007;446(7139):993–995.
- Proteintech. Post Translational Modification: an Overview. https://www.ptglab.com/news/blog/post-translational-modifications-an-overview Accessed August 20, 2025.
- Harmel R, Fiedler D. Features and regulation of non-enzymatic post-translational modifications. Nat Chem Biol. 2018;14(3):244–252.
- Müller MM. Post-translational modifications of protein backbones: unique functions, mechanisms, and challenges. Biochemistry. 2018;57(2):177–185.
- Klein T, et al. Proteolytic cleavage-mechanisms, function, and “omic” approaches for a near-ubiquitous posttranslational modification. Chem Rev. 2018;118(3):1137–1168.
- Savickas S, et al. Combinatorial degradomics: precision tools to unveil proteolytic processes in biological systems. Biochim Biophys Acta Proteins Proteom. 2020;1868(6):140392.
- Rogers LD, Overall CM. Proteolytic post-translational modification of proteins: proteomic tools and methodology. Mol Cell Proteomics. 2013;12(12):3532–3542.
- Hubbard SJ. The structural aspects of limited proteolysis of native proteins. Biochim Biophys Acta. 1998;1382(2):191–206.
- Goth CK, et al. Fine-tuning limited proteolysis: a major role for regulated site-specific O-Glycosylation. Trends Biochem Sci. 2018;43(4):269–284.
- Bischoff R, Schlüter H. Amino acids: chemistry, functionality and selected non-enzymatic post-translational modifications. J Proteomics. 2012;75(8):2275–2296.
- Rogers G, Simmonds D. Content of citrulline and other amino-acids in a protein of hair follicles. Nature. 1958;182(4629):186–187.
- Smith DG, Young EG. The combined amino acids in several species of marine algae. J Biol Chem. 1955;217(2):845–853.
- Lowden J, et al. The isolation and characterization of an acid-soluble protein from myelin. Can J Biochem. 1966;44(5):567–577.
- Finch P, et al. The presence of citrulline in a myelin protein fraction. FEBS Lett. 1971;15(2):145–148.
- Vossenaar ER, et al. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25(11):1106–1118.
- Tilvawala R, Thompson PR. Peptidyl arginine deiminases: detection and functional analysis of protein citrullination. Curr Opin Struct Biol. 2019;59:205–215.
- Xin F, Radivojac P. Post-translational modifications induce significant yet not extreme changes to protein structure. Bioinformatics. 2012;28(22):2905–2913.
- Jungblut PR, et al. The speciation of the proteome. Chem Cent J. 2008;2:16.
- Uy R, Wold F. Posttranslational Covalent Modification of Proteins: Only 20 amino acids are used in. protein synthesis, yet some 140” amino acids” are found in various proteins. Science. 1977;198(4320):890–896.
- Deribe YL, et al. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17(6):666–672.
- Walsh CT, et al. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl. 2005;44(45):7342–7372.
- Neeli I, et al. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180(3):1895–1902.
- Wang Y, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213.
- Tanikawa C, et al. Regulation of histone modification and chromatin structure by the p53-PADI4 pathway. Nat Commun. 2012;3(1):676.
- Cherrington BD, et al. Potential role for PAD2 in gene regulation in breast cancer cells. PLoS One. 2012;7(7):e41242.
- Zhang X, et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor α target gene activation. Proc Natl Acad Sci U S A. 2012;109(33):13331–13336.
- Yuzhalin AE. Citrullination in cancer. Cancer Res. 2019;79(7):1274–1284.
- Arita K, et al. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11(8):777–783.
- Snow AJ, et al. Phosphorylation-dependent interaction of tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (YWHA) with PADI6 following oocyte maturation in mice. Biol Reprod. 2008;79(2):337–347.
- Rohrbach A, ed. Physiological Pathways of PAD Activation and Citrullinated Eiptope Generation. Springer; 2014.
- Nakashima K, et al. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277(51):49562–49568.
- Cherrington BD, et al. Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones. PLoS One. 2010;5(7):e11768.
- Falcao AM, et al. PAD2-mediated citrullination contributes to efficient oligodendrocyte differentiation and myelination. Cell Rep. 2019;27(4):1090–1102.
- Millán-Zambrano G, et al. Histone post-translational modifications—cause and consequence of genome function. Nat Rev Genet. 2022;23(9):563–580.
- Cayman Chemical. Citrullination Research Tools. https://www.caymanchem.com/news/citrullination-research-tools Updated April 21, 2017. Accessed August 20, 2025.
- Zhang XG, et al. Genome-wide analysis reveals PADI4 cooperates with Elk-1 to activate c-Fos expression in breast cancer cells. PLoS Genetics. 2011;7(6):e1002112.
- Nakashima K, et al. PAD4 regulates proliferation of multipotent haematopoietic cells by controlling c-myc expression. Nat Commun. 2013;4(1):1836.
- Giambelluca MS, Gende OA. Hydrogen peroxide activates calcium influx in human neutrophils. Mol Cell Biochem. 2008;309(1-2):151–156.
- Bréchard S, Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008;84(5):1223–1237.
- Hayashi F, et al. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–2669.
- Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469.
- Sun B, et al. Citrullination of NF-κB p65 promotes its nuclear localization and TLR-induced expression of IL-1β and TNFα. Sci Immunol. 2017;2(12):eaal3062.
- Park J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138.
- Teijeira Á, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856–871.
- Jaillon S, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485–503.
- Chen Y, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320.
- Xu J, et al. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin Transl Oncol. 2017;19(8):989–996.
- Chae S, et al. Neutrophil-lymphocyte ratio predicts response to chemotherapy in triple-negative breast cancer. Curr Oncol. 2018;25(2):113–119.
- Albrengues J, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227.
- Yang L, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–138.
- Zhu YP, et al. NET formation is a default epigenetic program controlled by PAD4 in apoptotic neutrophils. Sci Adv. 2023;9(51):eadj1397.
- Hirschhorn D, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. 2023;186(7):1432–1447.
- Li Y, et al. Neutrophil extracellular traps induced by chemotherapy inhibit tumor growth in murine models of colorectal cancer. J Clin Invest. 2024;134(5):e175031.
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274.
- Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567.
- Lin H, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128(4):1708.
- Tang H, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 2018;128(2):580–588.
- Oh SA, et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer. 2020;1(7):681–691.
- Vossenaar ER, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63(4):373–381.
- Pitter MR, et al. PAD4 controls tumor immunity via restraining the MHC class II machinery in macrophages. Cell Rep. 2024;43(3):113942.
- Ting JPY, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109 Suppl(2):21–33.View this article via: PubMed Google Scholar
- Muhlethaler-Mottet A, et al. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8(2):157–166.
- Mishra N, et al. Cutting Edge: protein arginine deiminase 2 and 4 regulate NLRP3 inflammasome-dependent IL-1β maturation and ASC speck formation in macrophages. J Immunol. 2019;203(4):795–800.
- Wu Z, et al. Peptidylarginine deiminases 2 mediates caspase-1-associated lethality in pseudomonas aeruginosa pneumonia-induced sepsis. J Infect Dis. 2021;223(6):1093–1102.
- Shen Y, et al. Role of peptidyl arginine deiminase 4-dependent macrophage extracellular trap formation in type 1 diabetes pathogenesis. Diabetes. 2024;73(11):1862–1874.
- Bashar SJ, et al. Macrophage extracellular traps require peptidylarginine deiminase 2 and 4 and are a source of citrullinated antigens bound by rheumatoid arthritis autoantibodies. Front Immunol. 2024;15:1167362.
- Chen L, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9(6):597.
- Zhu S, et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 2021;12(11):984.
- Boe DM, et al. Extracellular traps and macrophages: new roles for the versatile phagocyte. J Leukoc Biol. 2015;97(6):1023–1035.
- Xia X, et al. The role of pyroptosis in cancer: pro-cancer or pro-”host”? Cell Death Dis. 2019;10(9):650.
- Tan Y, et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40(1):153.
- Deng H, et al. A novel selective inhibitor JBI-589 targets PAD4-mediated neutrophil migration to suppress tumor progression. Cancer Res. 2022;82(19):3561–3572.
- Sun B, et al. Reciprocal regulation of Th2 and Th17 cells by PAD2-mediated citrullination. JCI Insight. 2019;4(22):e129687.
- Liu Y, et al. Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight. 2018;3(23):e124729.
- Nishimura T, et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190(5):617–627.
- Kryczek I, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149.
- Kryczek I, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3(104):104ra100.
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–256.
- Loos T, et al. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood. 2008;112(7):2648–2656.
- Nagarsheth N, et al. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572.
- Ireland JM, Unanue ER. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med. 2011;208(13):2625–2632.
- Scherer HU, et al. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat Rev Rheumatol. 2018;14(3):157–169.
- Moon JS, et al. Cytotoxic CD8+ T cells target citrullinated antigens in rheumatoid arthritis. Nat Commun. 2023;14(1):319.
- Wu Z, et al. Peptidylarginine deiminase 2 in host immunity: current insights and perspectives. Front Immunol. 2021;12:761946.
- Geary B, et al. Peptidylarginine deiminase 2 citrullinates MZB1 and promotes the secretion of IgM and IgA. Front Immunol. 2023;14:1290585.
- Jang B, et al. Peptidylarginine deiminase inhibition impairs Toll-like receptor agonist-induced functional maturation of dendritic cells, resulting in the loss of T cell-proliferative capacity: a partial mechanism with therapeutic potential in inflammatory settings. J Leukoc Biol. 2015;97(2):351–362.
- Jang B, et al. The peptidylarginine deiminase inhibitor Cl-amidine suppresses inducible nitric oxide synthase expression in dendritic cells. Int J Mol Sci. 2017;18(11):2258.
- Xu W, et al. The role of nitric oxide in cancer. Cell Res. 2002;12(5):311–320.
- Fraszczak J, et al. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J Immunol. 2010;184(4):1876–1884.
- Krishnamurthy A, et al. Citrullination controls dendritic cell transdifferentiation into osteoclasts. J Immunol. 2019;202(11):3143–3150.
- Cuthbert GL, et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118(5):545–553.
- Wang Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306(5694):279–283.
- Mowen KA, et al. Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell. 2001;104(5):731–741.
- Inoue M, et al. Arginine methylation controls the strength of γc-family cytokine signaling in T cell maintenance. Nat Immunol. 2018;19(11):1265–1276.
- Mowen KA, et al. Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes. Mol Cell. 2004;15(4):559–571.
- Geoghegan V, et al. Comprehensive identification of arginine methylation in primary T cells reveals regulatory roles in cell signalling. Nat Commun. 2015;6(1):6758.
- Xu J, Richard S. Cellular pathways influenced by protein arginine methylation: Implications for cancer. Mol Cell. 2021;81(21):4357–4368.
- Wu Q, et al. Protein arginine methylation: from enigmatic functions to therapeutic targeting. Nat Rev Drug Discov. 2021;20(7):509–530.
- Infantino S, et al. Arginine methylation catalyzed by PRMT1 is required for B cell activation and differentiation. Nat Commun. 2017;8(1):891.
- Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65(1):8–24.
- Guccione E, Richard S. The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol. 2019;20(10):642–657.
- Lewallen DM, et al. Chemical proteomic platform to identify citrullinated proteins. ACS Chem Biol. 2015;10(11):2520–2528.
- Witalison E, et al. Protein arginine deiminases and associated citrullination: physiological functions and diseases associated with dysregulation. Curr Drug Targets. 2015;16(7):700–710.
- Mondal S, Thompson PR. Protein arginine deiminases (PADs): biochemistry and chemical biology of protein citrullination. Acc Chem Res. 2019;52(3):818–832.
- Association between subtypes of anti citrullinated peptide antibodies and lung damage in rheumatoid arthritis (ACPPPA). https://clinicaltrials.gov/study/NCT03832374 Accessed September 22, 2025.
- Impact of anti-citrullinated protein antibody status on treatment response and treatment persistence in participants with rheumatoid arthritis (RA) who are treated with abatacept or tumor necrosis factor inhibitors in Australia. https://clinicaltrials.gov/study/NCT03663829 Accessed September 22, 2025.
- A study on the prevalence of the modified citrullinated vimentin anti-body (Anti-MCV) in an Irish rheumatoid arthritis (RA) population and to assess the impact of anti-MCV and the anti-cyclic-citrullinated peptide antibody (Anti-CCP) status on the management of Irish patients with early RA. https://clinicaltrials.gov/study/NCT01078597 Accessed September 22, 2025.
- Mohanan S, et al. Potential role of peptidylarginine deiminase enzymes and protein citrullination in cancer pathogenesis. Biochem Res Int. 2012;2012(1):895343. View this article via: PubMed Google Scholar
- Knuckley B, et al. Profiling protein arginine deiminase 4 (PAD4): a novel screen to identify PAD4 inhibitors. Bioorg Med Chem. 2008;16(2):739–745.
- Lewallen DM, et al. A FluoPol-ABPP PAD2 high-throughput screen identifies the first calcium site inhibitor targeting the PADs. ACS Chem Biol. 2014;9(4):913–921.
- O’Dell J, et al. Treatment of early rheumatoid arthritis with minocycline or placebo: result of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1997;40(5):217–225.View this article via: PubMed Google Scholar
- Mangat P, et al. Bacterial and human peptidylarginine deiminases: targets for inhibiting the autoimmune response in rheumatoid arthritis? Arthritis Res Ther. 2010;12(3):209.
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. Adv Exp Med Biol. 2016;928:155–172.
- Yang C, et al. Peptidylarginine deiminases 4 as a promising target in drug discovery. Eur J Med Chem. 2021;226:113840.
- Dreyton CJ, et al. Insights into the mechanism of streptonigrin-induced protein arginine deiminase inactivation. Bioorg Med Chem. 2014;22(4):1362–1369.
- Lewis HD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11(3):189–191.
- Stone EM, et al. Inactivation of two diverse enzymes in the amidinotransferase superfamily by 2-chloroacetamidine: dimethylargininase and peptidylarginine deiminase. Biochemistry. 2005;44(42):13744–13752.
- Luo Y, et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry. 2006;45(39):11727–11736.
- Knuckley B, et al. Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry. 2010;49(23):4852–4863.
- Moscarello MA, et al. Inhibition of peptidyl-arginine deiminases reverses protein-hypercitrullination and disease in mouse models of multiple sclerosis. Dis Model Mech. 2013;6(2):467–478.View this article via: PubMed Google Scholar
- Zhu H, et al. Folded conformation, cyclic pentamer, nano-structure and PAD4 binding mode of YW3-56. J Phys Chem C Nanomater Interfaces. 2013;117(19):10070–10078.
- Wang S, et al. ATF4 Gene network mediates cellular response to the anticancer PAD inhibitor YW3-56 in triple-negative breast cancer cells. Mol Cancer Ther. 2015;14(4):877–888.
- Li M, et al. A novel peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 blocks formation of neutrophil extracellular traps and delays progression of multiple myeloma. Mol Cancer Ther. 2020;19(7):1530–1538.
- Humphries F, et al. Targeting STING oligomerization with small-molecule inhibitors. Proc Natl Acad Sci U S A. 2023;120(33):e2305420120.
- Willis VC, et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186(7):4396–4404.
- Willis VC, et al. Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis. Clin Exp Immunol. 2017;188(2):263–274.
- Muth A, et al. Development of a selective inhibitor of protein arginine deiminase 2. J Med Chem. 2017;60(7):3198–3211.
- Tian Y, et al. Peptidylarginine deiminase 2 has potential as both a biomarker and therapeutic target of sepsis. JCI Insight. 2020;5(20):e138873.
- Wu Z, et al. Inhibition of PAD2 improves survival in a mouse model of lethal LPS-induced endotoxic shock. Inflammation. 2020;43(4):1436–1445.
- Green RM, Thompson PR. Current insights into the role of citrullination in thrombosis. Curr Opin Chem Biol. 2023;75:102313.
- Zhou X, et al. Antibody discovery identifies regulatory mechanisms of protein arginine deiminase 4. Nat Chem Biol. 2024;20(6):742–750.
- Witalison EE, et al. Protein arginine deiminases and associated citrullination: physiological functions and diseases associated with dysregulation. Curr Drug Targets. 2015;16(7):700–710.
- Knuckley B, et al. A fluopol-ABPP HTS assay to identify PAD inhibitors. Chem Commun (Camb). 2010;46(38):7175–7177.
- Jones JE, et al. Synthesis and screening of a haloacetamidine containing library to identify PAD4 selective inhibitors. ACS Chem Biol. 2012;7(1):160–165.
- Slack JL, et al. Protein arginine deiminase 4: a target for an epigenetic cancer therapy. Cell Mol Life Sci. 2011;68(4):709–720.
- Witalison EE, et al. Molecular targeting of protein arginine deiminases to suppress colitis and prevent colon cancer. Oncotarget. 2015;6(34):36053–36062.
- Kim S, et al. Inhibition of protein arginine deiminase II suppresses retinoblastoma in orthotopic transplantation in mice. Oncol Rep. 2023;50(1):146.
- Zhu D, et al. PAD4 and its inhibitors in cancer progression and prognosis. Pharmaceutics. 2022;14(11):2414.
- Macia E, et al. Chlortetracycline, a novel arf inhibitor that decreases the Arf6-dependent invasive properties of breast cancer cells. Molecules. 2021;26(4):969.
- Tone M, et al. Tetracyclines enhance antitumor T-cell immunity via the Zap70 signaling pathway. J Immunother Cancer. 2024;12(4):e008334.
- Uysal-Onganer P, et al. Peptidylarginine deiminase isozyme-specific PAD2, PAD3 and PAD4 inhibitors differentially modulate extracellular vesicle signatures and cell invasion in two glioblastoma multiforme cell lines. Int J Mol Sci. 2020;21(4):1495.
- Wang B, et al. GSK484, an inhibitor of peptidyl arginine deiminase 4, increases the radiosensitivity of colorectal cancer and inhibits neutrophil extracellular traps. J Gene Med. 2023;25(9):e3530.
- Gajendran C, et al. Alleviation of arthritis through prevention of neutrophil extracellular traps by an orally available inhibitor of protein arginine deiminase 4. Sci Rep. 2023;13(1):3189.
- Ataie-Kachoie P, et al. Minocycline suppresses interleukine-6, its receptor system and signaling pathways and impairs migration, invasion and adhesion capacity of ovarian cancer cells: in vitro and in vivo studies. PLoS One. 2013;8(4):e60817.
- Dubey NK, et al. NSC 95397 suppresses proliferation and induces apoptosis in colon cancer cells through MKP-1 and the ERK1/2 pathway. Int J Mol Sci. 2018;19(6):1625.
- Lee SH, et al. Renal cell carcinoma is abrogated by p53 stabilization through transglutaminase 2 inhibition. Cancers (Basel). 2018;10(11):455.
- Wang Y, et al. Anticancer peptidylarginine deiminase (PAD) inhibitors regulate the autophagy flux and the mammalian target of rapamycin complex 1 activity. J Biol Chem. 2012;287(31):25941–25953.
- Zhu D, et al. Highly-tumor-targeted PAD4 inhibitors with PBA modification inhibit tumors in vivo by specifically inhibiting the PAD4-H3cit-NETs pathway in neutrophils. Eur J Med Chem. 2023;258:115619.
-
Version history
- Version 1 (October 15, 2025): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Metrics
Go to



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)




