Advertisement
Review
Open Access |  10.1172/JCI196346
10.1172/JCI196346
Mechanisms and clinical implications of gut-brain interactions
Zachary S. Lorsch1 and Rodger A. Liddle1,2,3
1Department of Medicine and
2Duke Institute for Brain Sciences, Duke University, Durham, North Carolina, USA.
3Department of Veterans Affairs Healthcare System, Durham, North Carolina, USA.
Address correspondence to: Rodger A. Liddle, Box 103859, 1033A Genome Science Research Building 1, 905 S. LaSalle Street, Durham, North Carolina 27710, USA. Email: rodger.liddle@duke.edu.
Find articles by Lorsch, Z. in: PubMed | Google Scholar
1Department of Medicine and
2Duke Institute for Brain Sciences, Duke University, Durham, North Carolina, USA.
3Department of Veterans Affairs Healthcare System, Durham, North Carolina, USA.
Address correspondence to: Rodger A. Liddle, Box 103859, 1033A Genome Science Research Building 1, 905 S. LaSalle Street, Durham, North Carolina 27710, USA. Email: rodger.liddle@duke.edu.
Find articles by
Liddle, R.
in:
PubMed
|
Google Scholar
|

Published January 2, 2026 - More info
J Clin Invest. 2026;136(1):e196346. https://doi.org/10.1172/JCI196346.
© 2026 Lorsch et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Connections between the digestive system and the brain have been postulated for over 2000 years. Despite this, only recently have specific mechanisms of gut-brain interaction been identified. Due in large part to increased interest in the microbiome, the wide use of incretin-based therapies (i.e., glucagon-like peptide 1 [GLP-1] receptor agonists), technological advancements, increased understanding of neuroimmunology, and the identification of a direct enteroendocrine cell–neural circuit, research in the past 10 years has made it abundantly clear that the gut-brain connection plays a role both in clinical disease as well as the actions of therapeutics. In this Review, we describe mechanisms by which the gut and brain communicate and highlight human and animal studies that implicate changes in gut-brain communication in disease states in gastroenterology, neurology, psychiatry, and endocrinology. Furthermore, we define how GLP-1 receptor agonists for obesity and guanylyl cyclase C agonists for irritable bowel syndrome leverage gut-brain mechanisms to improve patient outcomes. This Review illustrates the critical nature of gut-brain communication in human disease and the potential to target gut-brain pathways for therapeutic benefit.
-
Introduction
A common phrase attributed to the ancient Greek physician Hippocrates is, “all diseases begin in the gut.” While this gut-centric approach to medicine did not persist, recent research has implicated interactions between the digestive system, the gut microbiome, and other organ systems in wide-ranging pathophysiologies, including heart (1–3), lung (4), and chronic kidney diseases (5–7), cerebrovascular abnormalities (8), and cancer (9, 10). Due in part to a defined signaling pathway between the two organs via the vagus nerve (11), research on communication between the gastrointestinal tract and brain has given rise to the new field of gut-brain interactions.
Recognition of bidirectional communication between the gut and the brain is not new, as numerous historical examples, including William Beaumont’s 1833 finding that emotional arousal affects gastric function (12) and Ivan Pavlov’s Nobel Prize–winning work on the cephalic phase of digestion (13), suggested a physiologic connection between the two organs. However, only recently have the pathways and mechanisms underlying gut-brain interactions been described, and an array of disease phenotypes in which altered gut-brain communication is directly implicated been discovered. Furthermore, with the increasing use of incretin-based therapies (i.e., glucagon-like peptide 1 [GLP-1] receptor agonists) that leverage gut-brain mechanisms, gut-brain communication has become an important topic in both clinical and translational medicine. Here, we describe the four main mechanisms of communication between the gut and brain: hormonal, microbiome-mediated, immune-mediated, and direct signaling. We evaluate the role of disordered gut-brain interactions in the pathophysiology of several diseases (Table 1) and explain how the GLP-1 receptor agonists (e.g., semaglutide) for obesity and guanylyl cyclase C agonists (e.g., linaclotide) for irritable bowel syndrome (IBS) work through a gut-brain mechanism.
-
Mechanisms of gut-brain interaction
Interactions between the gut and the brain are bidirectional, involving multiple communicating systems including the gut and its contents, the microbiome, peripheral nerves, the enteric nervous system (ENS), autonomic nervous system, and the brain (14). Innervation of the gut is complex, with transcriptional profiles suggesting 21 neuronal and 3 glial cell subtypes in the ENS alone (15). The basic structure of the ENS consists of a submucosal plexus that controls mucosal processes and a myenteric plexus that controls movement, both of which are supported by interneurons and glia that refine function (16). In addition, intrinsic primary afferent neurons that project from the myenteric plexus to the subepithelial space receive sensory input from the gut epithelium via neurotransmitters (17). The colon is capable of peristalsis ex vivo, illustrating that extrinsic innervation is not required for the ENS to function. However, in vivo, the ENS is tightly connected to the autonomic nervous system via viscerofugal neurons that project out of the gut to connect to the sympathetic nervous system with direct feedback to the ENS to modulate motor activity (18). Separate from sympathetic pathways, the ENS communicates with the central nervous system (CNS) via the vagus and pelvic nerves (19). These nerve fibers are heterogeneous (20, 21), receive input from distinct sensory cells (22) and other neurons, and interact with ascending pathways. Consequently, gut-to-brain and brain-to-gut pathways involve interconnected networks of different sensory cells, neurons, interneurons, and glia.
Hormonal regulation of the gut-brain axis. The gut is the largest endocrine organ and secretes more than 30 individual hormones (23). These hormones are central in coordinating digestion and are one of four known processes that facilitate communication between the gut and the brain (Figure 1A). Hormonal signaling from gut to brain originates in enteroendocrine cells (EECs) that make up a small fraction of the gut epithelium. EECs are functionally defined by their hormonal expression, which varies according to location in the intestine and distribution along the crypt-villus axis (24). While the current naming convention is largely alphabetical (i.e., L cells) and mostly unrelated to function, there have been calls to rename these cells according to hormone secreted and location (25). It is not uncommon for EECs to secrete multiple hormones (26), and recent studies using intersectional genetics highlight a heterogeneous assortment of EEC types with differential effects on feeding behavior and gut motility (27, 28).
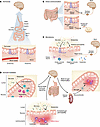 Figure 1
Figure 1Mechanisms of signaling between the gut and the brain. Information can be transmitted from the gut lumen to the brain in a variety of ways, but recent research has highlighted four distinct categories of signaling. (A) In hormonal signaling, hormones from the gut epithelium are either released into the bloodstream (endocrine) or locally (paracrine), where they act via receptors to exert an effect. Hormones act on receptors in the ENS and CNS (particularly the hypothalamus) to receive these signals. 5-HT, 5-hydroxytryptamine (serotonin). (B) In neuropod-mediated signaling, EECs form close connections that rapidly transmit information from the gut lumen to the CNS. In more proximal regions of the gut (i.e., stomach, small intestine), signals are typically transmitted via the vagus nerve and convey nutritive information. Neuropod signaling in more distal regions (i.e., colon) conveys information related to visceral pain and stretch, which are received by the brain via the dorsal root ganglia. (C) Gut microbiota produce local effects in the gut lumen that affect epithelial permeability and allow transmission of the microbiota or associated metabolites into the bloodstream. Some of these changes induce an inflammatory response. Alternatively, microbes or metabolites (such as short-chain fatty acids) act locally on receptors to modify cell function. (D) The gastrointestinal immune system surveils the gut lumen with resident T cells and neutrophils that are activated by microbes and their metabolites and convey signals to the brain. Responses can be modified via inflammation within the gastrointestinal tract, leading to increased permeability and allowing further immune interactions. Concurrently, top-down mechanisms have been described, including glucocorticoid-dependent activation of ENS microglia leading to inflammation within the gut epithelium.
While EECs can secrete hormones to affect digestive function in nearby organs (29), receptors for several gut hormones also exist in the CNS, including those for serotonin (30), cholecystokinin (CCK) (31), secretin (32), ghrelin (33), peptide YY (PYY) (34), and GLP-1 (35). Strikingly, some hormones have a more profound effect on gastrointestinal function when injected directly into the brain than when acting in the periphery (36). However, for peripheral hormones to exert a direct effect on the brain, they must overcome the blood-brain barrier. Hormones such as ghrelin and leptin are transported into the brain via specific transporter proteins (37, 38), but this is not a universal mechanism. In fact, serotonin, which exists as a neurotransmitter in the brain but is produced mostly in the gut (90% of total body content), does not cross into the CNS (39). In some cases, hormones can bypass the blood-brain barrier by binding to hormone receptors on circumventricular organs such the area postrema and subfornical organ (40). Alternatively, peripheral hormones can modify CNS activity through release of cytokines and nitric oxide from the blood-brain barrier itself (41).
Many of these same hormones can also act through peripheral nerves that signal to the brain. Notably, signal transduction through nerves such as the vagus, as well as afferent fibers with cell bodies in the dorsal root ganglia, represent a fundamental mechanism by which the CNS can receive information from the gut. In mice, the vagus nerve innervates the length of the gastrointestinal tract and contains receptors for hormones, including GLP-1, PYY, serotonin, and CCK (42). Vagal innervation is the greatest in the proximal intestine and declines across the length of the gastrointestinal tract, with possible differences in extent of innervation between human and mouse (43).
Direct connections. In the past decade, evidence has emerged that the gut communicates with peripheral nerves in a way that is distinct from hormonal signaling (Figure 1B). While it has been known for some time that EECs are electrically excitable (44), the discovery of neuropod cells, specialized EECs that contain podocyte-like processes near afferent nerve terminals and contain all necessary machinery for neurotransmission (45), suggested that this electrical excitability could convey information via a synaptic mechanism. Enterochromaffin cells are a large subset of EECs that are also electrically excitable, in close contact with nerve fibers, and can utilize neurotransmitters such as serotonin to directly modify signaling to communicate with the brain (46). Retrograde tracing techniques showed connections between neuropod cells and the brain in as little as a single synapse (47). However, recently it has been suggested that the distance between EECs and vagal and spinal afferents is too large to be classified as a synapse (48, 49), or that only a small subset of EECs form synaptic connections (50). Regardless, whether synaptic or paracrine, an EEC-neuronal connection distinct from hormonal signaling is a key mechanism of gut-brain communication.
The microbiome. Within the gut, the microbiome can influence brain function (Figure 1C). Microbiome composition is linked to diet (51) and can affect health (52–54). Gut microbes influence the CNS through hormone release, cytokine signaling, neurotransmitters, and release of bacterial byproducts that can either act within the gut or enter the systemic circulation (55). The microbiota and their metabolites have been shown to directly affect EECs (46, 56, 57), EEC-dependent activation of the vagus nerve (58) or sympathetic pathways (46), and hormone release (59). Thereby, the microbiome can modify gut-to-brain pathways to affect digestive function, CNS activity, and states of disease.
The immune system. Inflammation in peripheral organs, including the gastrointestinal tract, is linked to proinflammatory changes in the CNS (60, 61). Even in health, the gastrointestinal tract contains abundant immune cells that surveil the intraluminal contents (Figure 1D) (62, 63). In disease, cytokines can alter the permeability of the gastrointestinal epithelium, leading to exposure of gastrointestinal immune cells to alimentary contents (64). This is colloquially referred to as a “leaky gut,” and while the clinical implications of this are likely grossly overestimated (65), inflammation in the gastrointestinal tract can affect immune pathways and in turn gut-brain immune mechanisms. For example, a population of gut-derived T cells that are transcriptionally and functionally distinct from meningeal T cells migrate from the gastrointestinal tract to the paraventricular subfornical organ to regulate CNS homeostasis (66). Peripheral inflammation within the gastrointestinal tract can also be encoded by the brain. One study showed re-activation of gut-inflammation-responsive neurons in the insula reproduced gastrointestinal inflammatory patterns similar to the original peripheral insult (67). Taken together, these studies illustrate a role for immune cells in communicating signals from the gut to the brain and modifying CNS activity to respond to gut contents.
-
Gut-brain mechanisms of digestive disease
Disorders of gut-brain interaction. Over 40% of people worldwide are estimated to have a disorder of gut-brain interaction (DGBI) (68), which includes IBS and functional dyspepsia and negatively impacts quality of life (69). Previously referred to as functional gastrointestinal disorders, DGBIs were renamed by the Rome IV criteria in May 2016 to recognize underlying gut-brain pathophysiology (70). These conditions are unique in gastroenterology in that serology, imaging, and endoscopy in DGBIs are normal without any characteristic microscopic features on biopsy to establish a clinical diagnosis. Therefore, diagnosis of DGBI is based on symptom pattern with exclusion of alternative processes.
Clinically, the manifestations of DGBI are varied and include multiple different organs (esophagus, stomach, colon, biliary system), with symptoms ranging from dyspepsia to constipation and diarrhea. Multiple studies have shown frequent overlap between DGBI subtypes (i.e., IBS and functional dyspepsia) in the same patients and, when this occurs, the risk of comorbid psychiatric symptoms is greater (69, 71, 72). In fact, psychiatric comorbidities of DGBI are common (73), and psychiatric symptom scores are associated with a reduced likelihood to respond to any DGBI treatment, including neuromodulators (74).
Given this frequent overlap between DGBI and psychiatric symptoms, changes in circulating hormones, which affect both the gut and CNS, have been proposed to explain disease pathophysiology. For example, DGBIs are closely associated with psychological stress (75) to the extent that both adult stress and early life stress mouse models are used to simulate IBS (76). Accordingly, stress-dependent changes in corticotropin-releasing hormone (CRH) have been proposed as a mechanism for DGBIs by increasing intestinal permeability (77). Similarly, there is clinical and preclinical evidence that estrogen affects DGBI pathogenesis (78, 79), leading to the hypothesis that estrogen explains the higher prevalence of DGBIs in women (78, 80, 81).
In a study of postprandial hormone levels in patients with IBS and non-IBS controls, patients with IBS had increased postprandial gastrin and insulin and decreased postprandial ghrelin compared with non-IBS controls (82). This was associated with changes in gut motility, which is a common feature across numerous DGBIs. Other studies have identified changes in EEC abundance in IBS populations (83–85). However, the results of these studies are variable, and it is difficult to discern whether these effects are secondary to or causative of DGBI (86).
Pain is a central feature of many DGBIs and is felt to be secondary to underlying visceral hypersensitivity. In mice, chemogenetic activation and silencing of enterochromaffin cells with designer receptors exclusively activated by designer drugs (DREADDs) revealed that a serotonergic subset of EECs is directly involved in both the response to luminal irritants and the establishment of visceral hypersensitivity (87). Specifically, the effect of luminal irritants or colonic distension was mitigated when these cells were silenced, but activation of serotonergic enterochromaffin cells led to sex-dependent effects on visceral hypersensitivity via spinal afferents in the dorsal root ganglia (DRG). Interestingly, in this study, both increases and decreases in serotonin signaling promoted anxiety-like behavior. Other work has demonstrated similar phenomena, with a knockout of epithelial serotonin reuptake (increased serotonin) having opposite effects to epithelial blockade of serotonin synthesis (decreased serotonin), although these effects were dependent on the vagus nerve and not the DRG (88).
Enteroendocrine circuits in the small intestine have also been directly linked to visceral hypersensitivity in DGBIs, with selective knockout of the Gucy2c receptor on CCK-containing EECs increasing visceromotor response to rectal balloon distension (89). While CCK-containing EECs are present in more of the intestine in rodents than in humans, they are not present in the rectum (90) where the balloon was distended, suggesting the possibility of yet-to-be-identified circuits that facilitate small intestinal communication with other areas of the intestine. Interestingly, while knockout of the Gucy2c receptor in the intestinal epithelium affected p-ERK staining in the dorsal horn of the spinal cord (89), it is unknown whether the EECs containing the Gucy2c receptor also signal via the vagus to modify visceral pain.
Changes in the microbiome have been linked to disease subtypes across DGBIs (55, 91). Multiple studies demonstrate distinct microbial and microbe-associated metabolomic patterns in IBS (92–95). Interestingly, these features appear to correlate with known therapies. The microbial signature of IBS includes organisms that ferment carbohydrates (95); a diet low in fermentable sugars (low FODMAP diet) is a mainstay of IBS management, and adherence to a lowFODMAP diet appears to shift the microbial profile of patients with IBS toward that of non-IBS controls (94). Similarly, adherence to a combined Mediterranean and low FODMAP diet not only improved symptoms but reduced microbial byproducts: fecal short- and branched-chain fatty acids (96). Interestingly, related molecules (such as isovalerate; ref. 87) have been shown to affect visceral hypersensitivity through EECs. Together, these findings indicate that diet can promote specific microbial profiles that enhance/reduce production of microbial metabolites that affect gut-brain circuitry to elicit symptoms of IBS. Similar evidence for microbial changes exists in functional dyspepsia (97, 98), with a potential role for microbiome-induced changes in motility (98, 99) contributing to symptoms. Notably, microbial changes are closely associated with direct actions on EECs (99). Gut microbes may also promote EEC survival or proliferation, as fecal microbiota transplantation in IBS increases EEC abundance (85). However, to date, no unique microbial species has been proven causative in DGBI or been shown to modify individual EEC subtypes.
Although not fully established, multiple lines of evidence suggest a role for altered gut immunity in DGBIs. DGBIs are associated with increased intestinal permeability (77, 100) as well as low-level inflammation (101), with mast cells implicated as a possible driver of hypersensitivity in IBS (77, 102). Accordingly, stress-induced changes in food sensitivity, which overlap with the clinical features of IBS in many patients, induce pain in a mast cell–dependent matter (103). In experimental models, colonic inflammation stimulated monocyte and neutrophil migration to the brain, leading to anxiety-like behavior (104), a common clinical finding in IBS. Together, these studies indicate that DGBIs may involve changes in gut permeability due to low-level inflammation that either directly influences CNS activity or modifies gut-to-brain pathways.
Inflammatory bowel disease. Inflammatory bowel disease (IBD) is characterized by two disease phenotypes, Crohn’s disease and ulcerative colitis, that produce chronic inflammation in the intestine. As an autoimmune disease, the pathophysiology of IBD is not directly linked to changes in gut-brain signaling. However, recent studies have illustrated that gut-brain pathways play an important role in gut inflammation.
There is growing evidence that psychological stress promotes inflammation in the periphery (105). In turn, peripheral inflammation, as seen in IBD, can expose the CNS to inflammatory signals via stress-related breakdown of the blood-brain barrier (106). Clinically, perceived stress in IBD is associated with worse disease outcomes (107). To evaluate the role of stress-induced exacerbations in IBD, a recent study combined psychological stress with dextran sodium sulfate–induced colitis in mice (108). As in humans, psychological stress worsened colitis severity. This effect was mediated by CRH-dependent glucocorticoid release that prompted glial cells within the ENS to stimulate local monocyte populations to produce TNF-α. TNF-α is closely linked to IBD, and TNF-α inhibitors such as infliximab are a first-line biologic therapy for the disease. Chronic stress may also reduce the ability of the intestinal epithelium to regenerate, as chronic stress affects signaling from the dorsal motor nucleus of the vagus to the ENS, reducing stemness in intestinal crypts (109). Consequently, while IBD is not caused by abnormal gut-brain signaling, increased brain-to-gut activation of stress pathways exacerbates inflammation and reduces epithelial cell regeneration, leading to worsened clinical outcomes.
In addition to brain-to-gut pathways, EECs are important contributors to barrier function and gut immunity (110, 111). Therefore, changes in these cell populations could affect gut inflammation in IBD. Studies in both IBD patients (112) and mouse models of colitis (113) have identified increased EEC abundance with distinct hormonal changes in states of disease (114). For example, ghrelin levels are higher in active IBD than either quiescent IBD or healthy controls and have been proposed as part of a biomarker equation to noninvasively evaluate disease flares (115). GLP-1, which is increased in active IBD, normalizes upon disease quiescence (116). Increased vagal signaling is associated with an antiinflammatory response in the gut (117), and vagal tone is decreased in IBD (118). However, the specific efferent pathways driving this reduction in inflammation have yet to be identified, and while vagal stimulation has been proposed as an IBD therapy (119), clinical trials have not moved beyond the proof-of-concept stage.
Proinflammatory microbial changes are well described in IBD (120–122). The microbiome is closely linked to pro- and antiinflammatory states, which may be dependent on long-term dietary habits (123). Consequently, current guidance from the American Gastroenterological Association suggests that patients with IBD follow the Mediterranean diet (124), which is associated with microbial changes that reduce inflammation (125). Specific microbial changes in IBD have not been well established due to heterogeneity among studies (126). Even in studies of the same patient population, disease flares are associated with temporal variability in the microbiome signature (127), complicating global conclusions. Therefore, there is a need to better define gut microbiome changes and their upstream signaling changes, including the role of diet, to identify more specific therapeutic approaches.
In animal models of colitis, gut inflammation is present in specific neuronal populations in the insula, with re-activation of these neurons causing peripheral inflammation in the gastrointestinal tract (67). The exact mechanisms of this process are unclear, but studies suggest that colitis results in recruitment of immune cells to the brain (104) with the potential to directly affect brain activity (60).
Gastrointestinal motility disorders. Changes in the ENS or communication between the extrinsic nervous system and the ENS can lead to disorders across gastrointestinal organs, ranging from ineffective esophageal motility causing dysphagia, to dumping syndrome causing an autonomic response to carbohydrate-rich foods, to chronic constipation limiting regular defecation. Gastrointestinal hormones such as ghrelin and motilin are essential for gastric emptying (128, 129). In the small intestine, serotonin is critical to contraction and intermixing of digested contents (130), whereas GLP-1 can block normal peristalsis (131). In the colon, serotonin contributes to colonic motility (132). While direct hormonal actions on the gut could explain motility disorders independent of any communication with the brain, numerous neurological diseases, ranging from Parkinson’s disease (PD) to multiple sclerosis (MS), are associated with changes in gut motility (133). Accordingly, it is evident that CNS activity influences gut contractility and the ability to defecate. Experimentally in rats, stimulation of the locus coeruleus in the brainstem led to colonic contraction via noradrenergic and dopaminergic receptors in the lumbosacral defecation center (134), which is also affected by serotonin within the spinal cord (135). More recently, studies using DREADDs demonstrated top-down control of this defecation center from neurons in the brainstem that can modify both noxious stimuli and stress-induced defecation (136). Taken together, these studies demonstrate a role for the CNS in control of gut motility that may be involved in pathogenesis of motility disorders and represents a future target for therapeutics.
-
A gut-brain mechanism for neurologic disease
PD. PD is a motor disorder characterized by rigidity, decreased movement, and imbalance. The pathophysiology of PD is complex but involves loss of dopaminergic neurons in the substantia nigra through an interplay of neuroinflammation and α-synuclein accumulation. In addition to motor deficits, gastrointestinal symptoms are common in PD (137). Patients with PD are at higher risk for dysphagia, gastroparesis, constipation, and intestinal pseudo-obstruction even when compared to other neurological diseases such as Alzheimer’s disease (138). While neurological dysfunction could disrupt known top-down modulators of intestinal contraction that are known to play a role in motility (134), there is increasing evidence that the gut and associated gut-brain signaling are directly involved in the pathogenesis of PD. In support of this concept, epidemiological studies revealed that complete truncal vagotomy, but not the more limited highly selective vagotomy, was associated with reduced risk of PD (139).
α-Synuclein is produced in both the CNS and peripheral nervous system, including the gut, and increases in response to enteric infection (140) or inflammatory colitis (141). The Braak hypothesis postulates that PD pathology spreads in a predictable and progressive pattern (142), leading to the concept that α-synuclein spreads in a prion-like fashion from the gut to the brain via the vagus nerve (143, 144). In mice, injection of α-synuclein fibrils into the duodenal and pyloric muscularis induced α-synuclein pathology in the dorsal motor nucleus of the vagus and later other regions of the brain, including the substantial nigra (145). Vagotomy prevented this gut-to-brain spread. EECs contain α-synuclein (146), and by virtue of their connection with the vagus nerve, this established gut-to-brain circuit represents a possible pathway for the origin of PD. However alternative mechanisms of gut-to-brain transfer, including via immune cells, have also been proposed (147). A link between gut pathology and PD development in humans has also been shown, as patients with upper endoscopic findings of mucosal tears are more likely to develop PD later in life (148). However, it remains unclear whether this is causative or correlative, as it is unknown whether these tears lead to accumulation of α-synuclein or allow existing α-synuclein to transfer to the brain. Of note, the gut-to-brain model of PD is not without controversy, as there are also data to support brain-to-gut spread (120).
Within the gut, deposits of α-synuclein can have local consequences. In mice, transfer of human HLA-DRB1*15:01 and subsequent exposure to α-synuclein–derived epitopes leads to intestinal inflammation, a loss of enteric neurons, constipation, and weight loss (149). These data, combined with evidence of gastrointestinal inflammation leading to PD development (140, 141), support a possible neuroimmune role of PD pathogenesis. Thus, the clinical manifestations of PD in the gut, which can occur years before onset of motor symptoms, could be due to an inflammatory response to accumulating α-synuclein prior to CNS spread through the vagus. The microbiome may also play a role in this process, as the microbial profile of PD patients is distinct (150) and involves a preponderance of proinflammatory organisms, including those that dysregulate neuronal signaling and promote α-synuclein production (151).
MS. Although not as robust as PD, there is increasing evidence that changes in gut-brain signaling might also play a role in MS. MS is characterized by neuronal demyelination, leading to progressive neurologic dysfunction. MS is also associated with constipation. In experimental models of MS, autoantibodies against ENS and glia slowed intestinal transit (152).
The microbiome may also play a role in MS pathogenesis. In a study of 576 MS patients and 1152 household controls, patients with MS were found to have a distinct microbial and microbial metabolite profile that varied in response to disease-modifying treatments (153). Some studies have argued that the altered microbiome in MS causes disease (154), and others attempting to modify the microbiome have shown some success on improved disease course (155). The gut may also play a direct role in MS pathogenesis by initiating inflammatory pathways. Viewed by intravital imaging, autoreactive encephalitogenic T cell populations are activated in the lamina propria of the small intestine and subsequently adopt a Th17-like proinflammatory phenotype (156). This profile appeared to be dependent on both the microbiome and MHC II, as germ-free mice or MHC II antibodies greatly mitigated the inflammation. In support of a role for gut inflammation in initiating disease, molecular MRI evaluation of intestinal inflammation correlated with disease severity in a mouse model of autoimmune encephalitis (157). Together, these data suggest that MS may be dependent on gut inflammatory pathways, potentially in concert with the microbiome.
-
A gut-brain mechanism for psychiatric disease
Depression. The World Heath Organization estimates that 5.7% of adults worldwide suffer from depression (158). Depression, as well as depression-like symptoms, are closely comorbid with DGBIs (73), and changes in gut signaling pathways can produce anxiety and depression-like behavior (87, 88). Depression has also been linked to the microbiome, and fecal microbiota transplant from patients with depression to germ-free mice was sufficient to induce a depression-like phenotype (159) as well as an inflammatory hippocampal gene expression pattern (160). While CRH release following stress has been related to DGBI symptoms through increased intestinal permeability (77), this same permeability has been linked to social avoidance in a chronic social defeat stress model of depression (161). Gut-brain therapies have been attempted for major depressive disorder (MDD), including FDA-approved vagal stimulation for resistant disease (162), with recent clinical trials demonstrating at least some benefit (162). Thus, MDD pathophysiology may include top-down stress-responsive changes in intestinal permeability that facilitate intraluminal metabolites and the microbiome to affect CNS function, potentially involving the vagus nerve.
Schizophrenia. Like MDD, schizophrenia is associated with an altered gut microbiome profile and proinflammatory signaling pathways. Genome-wide association studies have demonstrated that schizophrenia and gastrointestinal diseases are closely related and share common gene variants linked to immune system function (163). Similarly, gut microbiome changes in patients with schizophrenia are closely related to proinflammatory pathways (164), although some multiomics analyses find less of an effect of the microbiome and more amino acid and lipid metabolism pathways affected (165). The mechanisms explaining these contributions are currently unknown.
-
Gut-brain mechanisms for obesity
Gut-brain signaling is essential to ingestive behavior. In 1950, a spontaneous mutation at The Jackson Laboratory led to the production of ob/ob mice, which exhibited weight up to four times that of a standard mouse (166). However, it was not until four decades later that the responsible gene mutation and its hormonal product, leptin, were identified (167). The discovery of ghrelin in rat stomach (168) led to the idea that opposing hormones modulate energy balance, with leptin promoting satiety and ghrelin stimulating food intake. A logical extension of this reasoning was that obesity resulted from excess ghrelin and reduced leptin. However, the relationship with BMI in patients is actually the opposite (positive correlation of BMI with leptin and negative correlation of BMI with ghrelin) (169). Furthermore, ghrelin, not leptin, increases with weight loss (170). This understanding led to the concept that resistance to these hormones exists in states of obesity (171). Another gut hormone, GLP-1, is secreted at lower levels in obese individuals (172, 173) and there is some evidence from human data favoring increased postprandial GLP-1 following weight loss (174). In addition, postprandial GLP-1 levels might play an important role in the success of bariatric surgery (175). Thus, hormonal signaling pathways appear to be related to obesity, but the exact contributions of each hormone are still being determined.
Recently, there has been increased recognition of the role of a direct gut-brain connection in guiding food intake. Diversity of both EECs as well as the vagal sensory neurons has been linked to specific components of feeding behavior (20, 28). Functionally, cells of the right vagus nerve form circuits with dopaminergic neurons in the substantia nigra that, when stimulated, facilitate a conditioned place preference toward the stimulated side (176). Consequently, activation of vagal afferents is rewarding, likely through dopaminergic release in the brain. This aspect of reward is essential to our understanding of obesity, as the high caloric macronutrients sugar (47, 177) and fat (178) have been shown to activate vagal pathways. While the vagal neurons involved in fat and sugar sensation are distinct, the combined ingestion of fat and sugar leads to more dopamine release in the nigrostriatum than either fat or sugar alone (179). Thus, the Western diet, which is high in both fats and sugars, might promote overeating and obesity through an additive effect on gut-brain reward pathways. In addition, neuropod cells activate the vagus nerve in response to intraluminal sugars via the neurotransmitter glutamate (47). These same cells respond to artificial sweeteners via a different neurotransmitter (ATP), and guide preferences for sugar over artificial sweetener following preconditioning (180). These findings suggest that direct gut-brain connections play a role in food choice.
Unsurprisingly, changes in the microbiome have been associated with obesity (181, 182). This is likely due at least in part to underlying differences in diet, which help to shape the microbiome (51). However, there are likely both causal and reactive components of the gut microbiome, so understanding the microbiome may assist in developing personalized interventions for weight loss (183). Accordingly, there is evidence for the microbiome-modulating efficacy of specific dietary strategies (184). Albeit with the same limitations of causation due to dietary changes, changes in the microbiome have been described in other eating disorders such as anorexia nervosa, bulimia nervosa, and binge eating disorder (185), with some evidence suggesting that altered microbial proteins can induce autoantibodies that affect neuroendocrine signaling (186).
-
Pharmacological targeting of gut-brain pathways
The extent of current pharmacologic agents that act on gut-brain pathways is unknown. However, two widely prescribed medications have been shown to be at least partially dependent on gut-brain mechanisms. Guanylyl cyclase C agonists have emerged as a target for visceral pain, with the finding that FDA-approved agonists such as linaclotide reduce pain in IBS. In an analysis of four separate randomized control trials, over 50% of patients with IBS with constipation treated with linaclotide reported at least a 30% reduction in abdominal pain (187). An explanation may lie in the discovery that GUCY2C neuropod hyperexcitability was reduced by linaclotide in vitro and correlated with reduction in visceral pain in vivo (Figure 2) (89). These data strongly suggest that directly targeting gut-brain signaling pathways via neuropod cells could help mitigate symptoms of visceral pain in DGBIs.
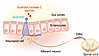 Figure 2
Figure 2Example of gut-brain directed pharmacotherapy. Guanylyl cyclase C agonists (e.g., linaclotide) bind to the GUCY2C receptor on neuropod cells to inhibit sensory neurons of the dorsal root ganglion and reduce visceral pain in IBS (89).
Since receiving FDA approval for treatment of obesity in 2014, GLP-1 receptor agonists have become a mainstay of obesity therapy. Recent evidence suggests these medications also may be effective in other brain-based disorders, including Alzheimer (188) and alcohol use disorder (189). In obesity, these drugs are highly effective, with some formulations achieving mean weight loss of over 20% in 72 months of follow-up (190). As GLP-1 is a gut hormone with receptors on the vagus (42), in the brain (35), and circumventricular organs (40), these medications directly mimic endogenous gut-brain incretin hormone pathways. GLP-1 has long been known to promote satiety and reduce food intake (191), although there is increasing evidence for accelerated metabolic activity (192). The exact mechanisms driving efficacy of GLP-1 receptor agonists in obesity are unknown, as the effects are widespread and include delayed gastric emptying and changes in blood glucose (193). Endogenous GLP-1 release within the brainstem reduces eating (194). While the vagus nerve contains neurons with receptors for GLP-1 as well as neurons that act on the brainstem to facilitate endogenous GLP-1 release, these two processes do not appear to overlap. Indeed, the vagal neurons that facilitate endogenous release of GLP-1 in the brainstem contain receptors for oxytocin but not GLP-1 (195). This suggests that there are parallel peripheral and central GLP-1 pathways that independently suppress appetite that could be simultaneously coopted for additive weight loss effects in humans. Accordingly, small studies using medications to target the brainstem preproglucagon neuronal pathway in concert with GLP-1 receptor agonists that do not target this pathway show promising effects (196).
-
Conclusion
Connections between the gastrointestinal tract and nervous system relay information about food, metabolites, and irritants within the gut lumen. While essential for homeostatic function, these pathways are altered in disease, with varying clinical presentations ranging from abdominal pain in IBS, to psychological distress in MDD, to constipation in PD. New technologies have enabled better understanding of gut-brain interactions, as well as how these pathways are implicated in disease and disease-modifying therapies. However, critical challenges lie ahead, including facilitating reproducibility, especially as it pertains to microbiome studies, translating studies to humans, and better clarifying the differing roles of rapid neuropod-based, paracrine, and hormonal EEC-based activities.
Nonetheless, our understanding of gut-to-brain signaling and its role in inflammation, stress, and metabolism has greatly expanded over the past decade. It is expected that future advances will lead to additional understanding of disease states and novel gut-brain–targeted therapeutics.
-
Funding support
This work is the result of NIH funding, in whole or in part, and is subject to the NIH Public Access Policy. Through acceptance of this federal funding, the NIH has been given a right to make the work publicly available in PubMed Central.
- NIH grants F32 DK142208 and T32 DK007568 (to ZSL).
- NIH grants DK124474 and DK120555 (to RAL).
- Aligning Science Across Parkinson’s award ASAP-020527 through the Michael J. Fox Foundation for Parkinson’s Research (to RAL).
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2026, Lorsch et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2026;136(1):e196346. https://doi.org/10.1172/JCI196346.
-
References
- Tang WHW, et al. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089–2105.
- Talmor-Barkan Y, et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat Med. 2022;28(2):295–302.
- Lin Y, et al. Bacterial extracellular vesicles in the initiation, progression and treatment of atherosclerosis. Gut Microbes. 2025;17(1):2452229.
- Druszczynska M, et al. The intriguing connection between the gut and lung microbiomes. Pathogens. 2024;13(11):1005.
- Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670.
- Ren F, et al. Genetic evidence supporting the causal role of gut microbiota in chronic kidney disease and chronic systemic inflammation in CKD: a bilateral two-sample Mendelian randomization study. Front Immunol. 2023;14:1287698.
- Fu S, et al. Investigating the role of gut microbiota in diabetic nephropathy through plasma proteome mediated analysis. Sci Rep. 2025;15(1):5457.
- Tang AT, et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature. 2017;545(7654):305–310.
- Helmink BA, et al. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388.
- Rahal Z, et al. Inflammation mediated by gut microbiome alterations promotes lung cancer development and an immunosuppressed tumor microenvironment. Cancer Immunol Res. 2024;12(12):1736–1752.
- Ma L, et al. The vagus nerve: An old but new player in brain-body communication. Brain Behav Immun. 2025;124:28–39.
- Beaumont W. The Physiology Of Digestion, With Experiments On The Gastric Juices. Goodrich; 1833.
- Pavlov I. The Work of the Digestive Glands. Charles Griffin; 1902.
- Gershon MD, Margolis KG. The gut, its microbiome, and the brain: connections and communications. J Clin Invest. 2021;131(18):e143768.
- Drokhlyansky E, et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182(6):1606–1622.
- Thomasi B, Gulbransen B. Mini-review: Intercellular communication between enteric glia and neurons. Neurosci Lett. 2023;806:137263.
- Clerc N, et al. Controlling the excitability of IPANs: a possible route to therapeutics. Curr Opin Pharmacol. 2002;2(6):657–664.
- Hibberd T, et al. Enteric control of the sympathetic nervous system. Adv Exp Med Biol. 2022;1383:89–103.
- Furness JB, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71.
- Bai L, et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179(5):1129–1143.
- Wolfson RL, et al. DRG afferents that mediate physiologic and pathologic mechanosensation from the distal colon. Cell. 2023;186(16):3368–3385.
- Alcaino C, et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115(32):7632–7641.
- Ahlman H, Nilsson O. The gut as the largest endocrine organ in the body. Ann Oncol. 2001;12 Suppl 2:S63–S68.
- Billing LJ, et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice - Identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol Metab. 2019;29:158–169.
- Helander HF, Fndriks L. The enteroendocrine “letter cells” - time for a new nomenclature? Scand J Gastroenterol. 2012;47(1):3–12.
- Egerod KL, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795.
- Hayashi M, et al. Enteroendocrine cell lineages that differentially control feeding and gut motility. Elife. 2023;12:e78512.
- Bai L, et al. Enteroendocrine cell types that drive food reward and aversion. Elife. 2022;11:e74964.
- Bany Bakar R, et al. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat Rev Gastroenterol Hepatol. 2023;20(12):784–796.
- Salvan P, et al. Serotonin regulation of behavior via large-scale neuromodulation of serotonin receptor networks. Nat Neurosci. 2023;26(1):53–63.
- Hill DR, et al. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987;7(9):2967–2976.
- Tóth ZE, et al. Distribution of secretin receptors in the rat central nervous system: an in situ hybridization study. J Mol Neurosci. 2013;50(1):172–178.
- Gnanapavan S, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988.
- Inui A, et al. Characterization of peptide YY receptors in the brain. Endocrinology. 1989;124(1):402–409.
- Heppner KM, et al. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology. 2015;156(1):255–267.
- Mutt V, et al. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979;25(20):1703–1707.
- Rhea EM, et al. Ghrelin transport across the blood-brain barrier can occur independently of the growth hormone secretagogue receptor. Mol Metab. 2018;18:88–96.
- Banks WA, et al. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17(2):305–311.
- El-Merahbi R, et al. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589(15):1728–1734.
- Benarroch EE. Circumventricular organs: receptive and homeostatic functions and clinical implications. Neurology. 2011;77(12):1198–1204.
- Banks WA. The blood-brain barrier: connecting the gut and the brain. Regul Pept. 2008;149(1-3):11–14.
- Lansbury EL, et al. Neurons co-expressing GLP-1, CCK, and PYY receptors particularly in right nodose ganglion and innervating entire GI tract in mice. Int J Mol Sci. 2025;26(5):2053.
- Wang L, Taché Y. The parasympathetic and sensory innervation of the proximal and distal colon in male mice. Front Neuroanat. 2024;18:1422403.
- Rogers GJ, et al. Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. J Physiol. 2011;589(pt 5):1081–1093.
- Bohórquez DV, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125(2):782–786.
- Bellono NW, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198.
- Kaelberer MM, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408):eaat5236.
- Dodds KN, et al. The gut-brain axis: spatial relationship between spinal afferent nerves and 5-HT-containing enterochromaffin cells in mucosa of mouse colon. Am J Physiol Gastrointest Liver Physiol. 2022;322(5):523–533.
- Spencer NJ, et al. Identification of vagal afferent nerve endings in the mouse colon and their spatial relationship with enterochromaffin cells. Cell Tissue Res. 2024;396(3):313–327.
- Cao N, et al. Limited evidence for anatomical contacts between intestinal GLP-1 cells and vagal neurons in male mice. Sci Rep. 2024;14(1):23666.
- Asnicar F, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–332.
- Hindle VK, et al. Microbiota-focused dietary approaches to support health: a systematic review. J Nutr. 2025;155(2):381–401.
- Ross FC, et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol. 2024;22(11):671–686.
- Garcia-Mantrana I, et al. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. 2018;9:890.
- Margolis KG, et al. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–1501.
- Ye L, et al. High fat diet induces microbiota-dependent silencing of enteroendocrine cells. Elife. 2019;8:e48479.
- Liu WW, et al. A gut sense for a microbial pattern regulates feeding. Nature. 2025;645(8081):729–736.
- Ye L, et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29(2):179–196.
- Chao J, et al. Gut microbiome regulation of gut hormone secretion. Endocrinology. 2025;166(4):bqaf004.
- Mitchell J, et al. Chronic intestinal inflammation suppresses brain activity by inducing neuroinflammation in mice. Am J Pathol. 2022;192(1):72–86.
- D’Mello C, et al. P-selectin-mediated monocyte-cerebral endothelium adhesive interactions link peripheral organ inflammation to sickness behaviors. J Neurosci. 2013;33(37):14878–14888.
- Hegazy AN, et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology. 2017;153(5):1320–1337.
- Knoop KA, et al. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015;8(1):198–210.
- Meyer F, et al. Cytokines and intestinal epithelial permeability: A systematic review. Autoimmun Rev. 2023;22(6):103331.
- Lacy BE, et al. Leaky gut syndrome: myths and management. Gastroenterol Hepatol (N Y). 2024;20(5):264–272.View this article via: PubMed Google Scholar
- Yoshida TM, et al. The subfornical organ is a nucleus for gut-derived T cells that regulate behaviour. Nature. 2025;643(8071):499–508.
- Koren T, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184(24):5902–5915.
- Sperber AD, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology. 2021;160(1):99–114.
- Knowles SR, et al. Negative impact of disorders of gut-brain interaction on health-related quality of life: results from the Rome Foundation global epidemiology survey. Gastroenterology. 2023;164(4):655–668.
- Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23(2):151–163.
- Sperber AD, et al. Greater overlap of Rome IV disorders of gut-brain interactions leads to increased disease severity and poorer quality of life. Clin Gastroenterol Hepatol. 2022;20(5):945–956.
- Fairlie T, et al. Overlap of disorders of gut-brain interaction: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(7):646–659.
- Zia JK, et al. Risk factors for abdominal pain-related disorders of gut-brain interaction in adults and children: a systematic review. Gastroenterology. 2022;163(4):995–1023.
- Madva EN, et al. Psychiatric comorbidities among adult patients with disorders of gut-brain interaction: Prevalence and relationships to treatment outcomes. Neurogastroenterol Motil. 2023;35(2):e14493.
- Chan Y, et al. The temporal relationship of daily life stress, emotions, and bowel symptoms in irritable bowel syndrome—diarrhea subtype: A smartphone-based experience sampling study. Neurogastroenterol Motil. 2019;31(3):e13514.
- Meerveld BG, Johnson AC. Mechanisms of stress-induced visceral pain. J Neurogastroenterol Motil. 2018;24(1):7–18.
- Vanuytsel T, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299.
- Sarnoff RP, et al. Sex differences, menses-related symptoms and menopause in disorders of gut–brain interaction. Neurogastroenterol Motil. 2025;37(2):e14977.
- Accarie A, et al. Estrogens play a critical role in stress-related gastrointestinal dysfunction in a spontaneous model of disorders of gut–brain interaction. Cells. 2022;11(7):1214.
- Mulak A, et al. Sex- and gender-related differences in the prevalence and burden of disorders of gut-brain interaction in Poland. Neurogastroenterol Motil. 2023;35(6):e14568.
- Kovács DB, et al. Prevalence, epidemiology and associated healthcare burden of Rome IV irritable bowel syndrome and functional dyspepsia in the adult population of Gibraltar. BMJ Open Gastroenterol. 2022;9(1):e000979.
- Furgała A, et al. Postprandial effect of gastrointestinal hormones and gastric activity in patients with irritable bowel syndrome. Sci Rep. 2023;13(1):9420.
- El-Salhy M, et al. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23(6):703–707.
- El-Salhy M, Gilja OH. Abnormalities in ileal stem, neurogenin 3, and enteroendocrine cells in patients with irritable bowel syndrome. BMC Gastroenterol. 2017;17(1):90.
- Mazzawi T, et al. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand J Gastroenterol. 2022;57(7):792–796.
- Barton JR, et al. Enteroendocrine cell regulation of the gut-brain axis. Front Neurosci. 2023;17:1272955.
- Bayrer JR, et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature. 2023;616(7955):137–142.
- Hung LY, et al. Intestinal epithelial serotonin as a novel target for treating disorders of gut-brain interaction and mood. Gastroenterology. 2025;168(4):754–768.
- Barton JR, et al. Intestinal neuropod cell GUCY2C regulates visceral pain. J Clin Invest. 2023;133(4):e165578.
- Fakhry J, et al. Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell Tissue Res. 2017;369(2):245–253.
- Shin A, et al. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol. 2019;17(2):256–274.
- Agnello M, et al. Gut microbiome composition and risk factors in a large cross-sectional IBS cohort. BMJ Open Gastroenterol. 2020;7(1):e000345.
- Han L, et al. Altered metabolome and microbiome features provide clues in understanding irritable bowel syndrome and depression comorbidity. ISME J. 2022;16(4):983–996.
- Vervier K, et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut. 2022;71(9):1821–1830.
- Jacobs JP, et al. Multi-omics profiles of the intestinal microbiome in irritable bowel syndrome and its bowel habit subtypes. Microbiome. 2023;11(1):5.
- Kasti AN, et al. Clinical Trial: A Mediterranean low-FODMAP diet alleviates symptoms of non-constipation IBS—randomized controlled study and volatomics analysis. Nutrients. 2025;17(9):1545.
- Brown G, et al. Role of the duodenal microbiota in functional dyspepsia. Neurogastroenterol Motil. 2022;34(11):e14372.
- Shanahan ER, et al. Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in functional dyspepsia. Gut. 2023;72(5):929–938.
- Fukui H, et al. Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J Neurogastroenterol Motil. 2018;24(3):367–386.
- Grover M, et al. Intestinal permeability in disorders of gut-brain interaction: from bench to bedside. Gastroenterology. 2024;168(3):480–495.
- Yuan Y, et al. Low-level inflammation, immunity, and brain-gut axis in IBS: unraveling the complex relationships. Gut Microbes. 2023;15(2):2263209.
- Aguilera-Lizarraga J, et al. Immune activation in irritable bowel syndrome: what is the evidence? Nat Rev Immunol. 2022;22(11):674–686.
- Aguilera-Lizarraga J, et al. Psychological stress-induced local immune response to food antigens increases pain signaling across the gut in mice. Gastroenterology. 2025;169(1):104–118.
- Cluny NL, et al. Recruitment of α4β7 monocytes and neutrophils to the brain in experimental colitis is associated with elevated cytokines and anxiety-like behavior. J Neuroinflammation. 2022;19(1):73.
- Chan KL, et al. Central regulation of stress-evoked peripheral immune responses. Nat Rev Neurosci. 2023;24(10):591–604.
- Menard C, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20(12):1752–1760.
- Sauk JS, et al. High perceived stress is associated with increased risk of ulcerative colitis clinical flares. Clin Gastroenterol Hepatol. 2023;21(3):741–749.
- Schneider KM, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186(13):2823–2838.
- Zhang G, et al. Vagal pathway activation links chronic stress to decline in intestinal stem cell function. Cell Stem Cell. 2025;32(5):778–794.
- Nwako JG, McCauley HA. Enteroendocrine cells regulate intestinal homeostasis and epithelial function. Mol Cell Endocrinol. 2024;593:112339.
- Worthington JJ, et al. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018;11(1):3–20.
- El-Salhy M, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242(5):413–419.
- Hernández-Trejo AJ, et al. The pro-inflammatory cytokines IFNγ/TNFα increase chromogranin A-positive neuroendocrine cells in the colonic epithelium. Biochem J. 2016;473(21):3805–3818.
- El-Salhy M, et al. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J Gastroenterol. 2017;23(28):5068–5085.
- Khalaji A, et al. Unveiling the ghrelin and obestatin roles in inflammatory bowel diseases: a systematic review and meta-analysis assessing their pathogenic implications and biomarker utility. Inflamm Bowel Dis. 2024;30(4):629–640.
- Keller J, et al. Gastric emptying and disease activity in inflammatory bowel disease. Eur J Clin Invest. 2015;45(12):1234–1242.
- Stavely R, et al. Divergent adaptations in autonomic nerve activity and neuroimmune signaling associated with the severity of inflammation in chronic colitis. Inflamm Bowel Dis. 2022;28(8):1229–1243.
- Bonaz B, et al. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452.
- Hesampour F, et al. Brain-gut axis: invasive and noninvasive vagus nerve stimulation, limitations, and potential therapeutic approaches. Inflamm Bowel Dis. 2024;30(3):482–495.
- Schirmer M, et al. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17(8):497–511.
- Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652–2664.
- Hu Y, et al. Disturbances of the gut microbiota and microbiota-derived metabolites in inflammatory bowel disease. Nutrients. 2022;14(23):5140.
- Bolte LA, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70(7):1287–1298.
- Hashash JG, et al. AGA clinical practice update on diet and nutritional therapies in patients with inflammatory bowel disease: expert review. Gastroenterology. 2024;166(3):521–532.
- Turpin W, et al. Mediterranean-like dietary pattern associations with gut microbiome composition and subclinical gastrointestinal inflammation. Gastroenterology. 2022;163(3):685–698.
- Pittayanon R, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology. 2020;158(4):930–946.
- Lloyd-Price J, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662.
- Camilleri M. Gastrointestinal hormones and regulation of gastric emptying. Curr Opin Endocrinol Diabetes Obes. 2019;26(1):3–10.
- Levin F, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–3302.
- Ellis M, et al. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304(8):749–761.
- Tolessa T, et al. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. J Clin Invest. 1998;102(4):764–774.
- Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil. 2015;27(7):899–905.
- Camilleri M. Gastrointestinal motility disorders in neurologic disease. J Clin Invest. 2021;131(4):e143771.
- Nakamori H, et al. Roles of the noradrenergic nucleus locus coeruleus and dopaminergic nucleus A11 region as supraspinal defecation centers in rats. Am J Physiol Gastrointest Liver Physiol. 2019;317(4):G545–G555.
- Nakamori H, et al. Exogenous serotonin regulates colorectal motility via the 5-HT2 and 5-HT3 receptors in the spinal cord of rats. Neurogastroenterol Motil. 2018;30(3):e13183. View this article via: CrossRef Google Scholar
- Sawamura T, et al. Essential roles of the hypothalamic A11 region and the medullary raphe nuclei in regulation of colorectal motility in rats. Am J Physiol Gastrointest Liver Physiol. 2023;324(6):466–475.
- Travagli RA, et al. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2020;17(11):673–685.
- Konings B, et al. Gastrointestinal syndromes preceding a diagnosis of Parkinson’s disease: testing Braak’s hypothesis using a nationwide database for comparison with Alzheimer’s disease and cerebrovascular diseases. Gut. 2023;72(11):2103–2111.
- Svensson E, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529.
- Stolzenberg E, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9(5):456–463.
- Casini A, et al. TNBS colitis induces architectural changes and alpha-synuclein overexpression in mouse distal colon: A morphological study. Cell Tissue Res. 2024;399(2):247–265.
- Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211.View this article via: PubMed Google Scholar
- Olanow CW, Prusiner SB. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28(1):31–40.
- Braak H, et al. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110(5):517–536.
- Kim S, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103(4):627–641.
- Chandra R, et al. Gut mucosal cells transfer α-synuclein to the vagus nerve. JCI Insight. 2023;8(23):e172192.
- McFleder RL, et al. Brain-to-gut trafficking of alpha-synuclein by CD11c+ cells in a mouse model of Parkinson’s disease. Nat Commun. 2023;14(1):7529.
- Chang JJ, et al. Upper gastrointestinal mucosal damage and subsequent risk of Parkinson disease. JAMA Netw Open. 2024;7(9):e2431949.
- Garretti F, et al. Interaction of an α-synuclein epitope with HLA-DRB1*15:01 triggers enteric features in mice reminiscent of prodromal Parkinson’s disease. Neuron. 2023;111(21):3397–3413.View this article via: CrossRef Google Scholar
- Romano S, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27.
- Wallen ZD, et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun. 2022;13(1):6958.
- Spear ET, et al. Altered gastrointestinal motility involving autoantibodies in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neurogastroenterol Motil. 2018;30(9):e13349.
- Zhou X, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467–3486.
- Zancan V, et al. Gut microbiota composition is causally linked to multiple sclerosis: a mendelian randomization analysis. Microorganisms. 2024;12(7):1476.
- Ghezzi L, et al. Targeting the gut to treat multiple sclerosis. J Clin Invest. 2021;131(13):e143774.
- Bauer IJ, et al. Visualizing the activation of encephalitogenic T cells in the ileal lamina propria by in vivo two-photon imaging. Proc Natl Acad Sci U S A. 2023;120(30):e2302697120.
- Baudron E, et al. Intestinal MAdCAM-1 imaging as biomarker for prognostic in murine models of multiple sclerosis. Brain Behav Immun. 2024;119:381–393.
- World Health Organization. Depressive Disorder (Depression). https://www.who.int/news-room/fact-sheets/detail/depression Updated August 29, 2025. Accessed October 7, 2025.
- Zheng P, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–796.
- Chen X, et al. A comprehensive analysis of the differential expression in the hippocampus of depression induced by gut microbiota compared to traditional stress. Gene. 2024;927:148633.
- Russo S, et al. Stress-activated brain-gut circuits disrupt intestinal barrier integrity and social behaviour [preprint]. https://doi.org/10.21203/rs.3.rs-3459170/v1 Posted on Res Sq October 27, 2023.
- Conway CR, et al. Vagus nerve stimulation in treatment-resistant depression: A one-year, randomized, sham-controlled trial. Brain Stimul. 2024;18(3):676–689.
- Xie Y, et al. Shared genetic architecture among gastrointestinal diseases, schizophrenia, and brain subcortical volumes. Schizophr Bull. 2024;50(5):1243–1254.
- Yuan X, et al. Intestinal mycobiota dysbiosis associated inflammation activation in chronic schizophrenia. Behav Brain Res. 2024;472:115149.
- Qi D, et al. Unveiling the gut microbiota blueprint of schizophrenia: a multilevel omics approach. Front Psychiatry. 2024;15:1452604.
- Ingalls AM, et al. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318.
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432.
- Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660.
- Monti V, et al. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J Am Diet Assoc. 2006;106(6):822–828.
- Coutinho SR, et al. Impact of weight loss achieved through a multidisciplinary intervention on appetite in patients with severe obesity. Am J Physiol Endocrinol Metab. 2018;315(1):91–98.
- Cui H, et al. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol. 2017;13(6):338–351.
- Muscelli E, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57(5):1340–1348.
- Ranganath LR, et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38(6):916–919.
- Otten J, et al. Postprandial levels of GLP-1, GIP and glucagon after 2 years of weight loss with a Paleolithic diet: a randomised controlled trial in healthy obese women. Eur J Endocrinol. 2019;180(6):417–427.
- Çalık Başaran N, et al. Post metabolic bariatric surgery weight regain: the importance of GLP-1 levels. Int J Obes (Lond). 2024;49(3):412–417.
- Han W, et al. A neural circuit for gut-induced reward. Cell. 2018;175(3):665–678.View this article via: CrossRef Google Scholar
- Tan HE, et al. The gut-brain axis mediates sugar preference. Nature. 2020;580(7804):511–516.
- Li M, et al. Gut-brain circuits for fat preference. Nature. 2022;610(7933):722–730.
- McDougle M, et al. Separate gut-brain circuits for fat and sugar reinforcement combine to promote overeating. Cell Metab. 2024;36(2):393–407.
- Buchanan KL, et al. The preference for sugar over sweetener depends on a gut sensor cell. Nat Neurosci. 2022;25(2):191–200.
- Aron-Wisnewsky J, et al. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology. 2021;160(2):573–599.
- Van Hul M, Cani PD. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat Rev Endocrinol. 2023;19(5):258–271.
- Carmody RN, Bisanz JE. Roles of the gut microbiome in weight management. Nat Rev Microbiol. 2023;21(8):535–550.
- Ben-Yacov O, et al. Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: a diet intervention in pre-diabetes. Gut. 2023;72(8):1486–1496.
- Marano G, et al. Gut microbiota in women with eating disorders: a new frontier in pathophysiology and treatment. Nutrients. 2025;17(14):2316.
- Smitka K, et al. Current aspects of the role of autoantibodies directed against appetite-regulating hormones and the gut microbiome in eating disorders. Front Endocrinol. 2021;12:613983.
- Brenner DM, et al. Linaclotide reduced response time for irritable bowel syndrome with constipation symptoms: analysis of 4 randomized controlled trials. Am J Gastroenterol. 2023;118(5):872–879.
- Liang Y, et al. Clinical Evidence for GLP-1 receptor agonists in alzheimer’s disease: a systematic review. J Alzheimers Dis Rep. 2024;8(1):777–789.
- Hendershot CS, et al. Once-weekly semaglutide in adults with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry. 2025;82(4):395–405.
- Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216.
- Flint A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520.
- O’Donnell C, et al. GLP-1 therapy increases visceral adipose tissue metabolic activity: lessons from a randomized controlled trial in obstructive sleep apnea. Obesity (Silver Spring). 2024;32(11):2077–2081.
- Umapathysivam MM, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes. 2014;63(2):785–790.
- Holt MK, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21–33.
- Brierley DI, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021;3(2):258–273.
- Wagner S, et al. Obesity medication lorcaserin activates brainstem GLP-1 neurons to reduce food intake and augments GLP-1 receptor agonist induced appetite suppression. Mol Metab. 2023;68:101665.
-
Version history
- Version 1 (January 2, 2026): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Metrics
Go to
- Top
- Abstract
- Introduction
- Mechanisms of gut-brain interaction
- Gut-brain mechanisms of digestive disease
- A gut-brain mechanism for neurologic disease
- A gut-brain mechanism for psychiatric disease
- Gut-brain mechanisms for obesity
- Pharmacological targeting of gut-brain pathways
- Conclusion
- Funding support
- Footnotes
- References
- Version history



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)



