Citation Information: J Clin Invest. 2019;129(9):3941-3951. https://doi.org/10.1172/JCI127511.
Abstract
Nature exploits cage-like proteins for a variety of biological purposes, from molecular packaging and cargo delivery to catalysis. These cage-like proteins are of immense importance in nanomedicine due to their propensity to self-assemble from simple identical building blocks to highly ordered architecture and the design flexibility afforded by protein engineering. However, delivery of protein nanocages to the renal tubules remains a major challenge because of the glomerular filtration barrier, which effectively excludes conventional size nanocages. Here, we show that DNA-binding protein from starved cells (Dps) — the extremely small archaeal antioxidant nanocage — is able to cross the glomerular filtration barrier and is endocytosed by the renal proximal tubules. Using a model of endotoxemia, we present an example of the way in which proximal tubule–selective Dps nanocages can limit the degree of endotoxin-induced kidney injury. This was accomplished by amplifying the endogenous antioxidant property of Dps with addition of a dinuclear manganese cluster. Dps is the first-in-class protein cage nanoparticle that can be targeted to renal proximal tubules through glomerular filtration. In addition to its therapeutic potential, chemical and genetic engineering of Dps will offer a nanoplatform to advance our understanding of the physiology and pathophysiology of glomerular filtration and tubular endocytosis.
Authors
Masaki Uchida, Bernhard Maier, Hitesh Kumar Waghwani, Ekaterina Selivanovitch, S. Louise Pay, John Avera, EJun Yun, Ruben M. Sandoval, Bruce A. Molitoris, Amy Zollman, Trevor Douglas, Takashi Hato
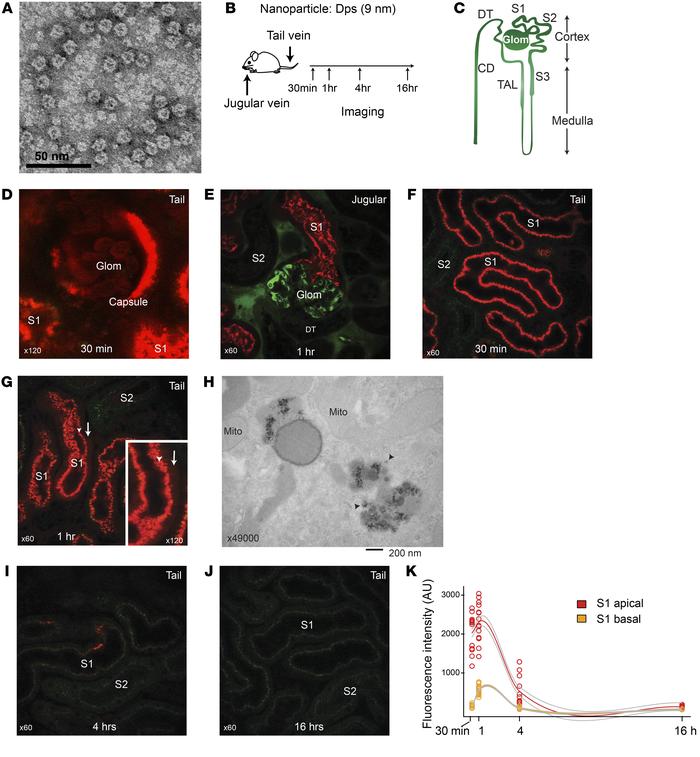
Figure 1
Dps is filtered through glomeruli and endocytosed by proximal tubules.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)