Abstract
A population of NK cells expressing the activating receptor NKG2C and the maturation marker CD57 expands in response to human CMV (HCMV) infection. CD3–CD56dimCD57+NKG2C+ NK cells are similar to CD8+ memory T cells with rapid and robust effector function upon restimulation, persistence, and epigenetic remodeling of the IFNG locus. Chronic antigen stimulation drives CD8+ memory T cell proliferation, while also inducing genome-wide epigenetic reprograming and dysfunction. We hypothesized that chronic stimulation could similarly induce epigenetic reprograming and dysfunction in NK cells. Here, we show that chronic stimulation of adaptive NK cells through NKG2C using plate-bound agonistic Abs in combination with IL-15 drove robust proliferation and activation of CD3–CD56dimCD57+NKG2C+ NK cells, while simultaneously inducing high expression of the checkpoint inhibitory receptors LAG-3 and PD-1. Marked induction of checkpoint inhibitory receptors was also observed on the surface of adaptive NK cells cocultured with HCMV-infected endothelial cells. Chronically stimulated adaptive NK cells were dysfunctional when challenged with tumor targets. These cells exhibited a pattern of epigenetic reprograming, with genome-wide alterations in DNA methylation. We believe our study has important implications for cancer immunotherapy and propose that exhausted NK cells could be targeted with inhibitory checkpoint receptor blockade.
Authors
Aimee Merino, Bin Zhang, Philip Dougherty, Xianghua Luo, Jinhua Wang, Bruce R. Blazar, Jeffrey S. Miller, Frank Cichocki
Figure 2
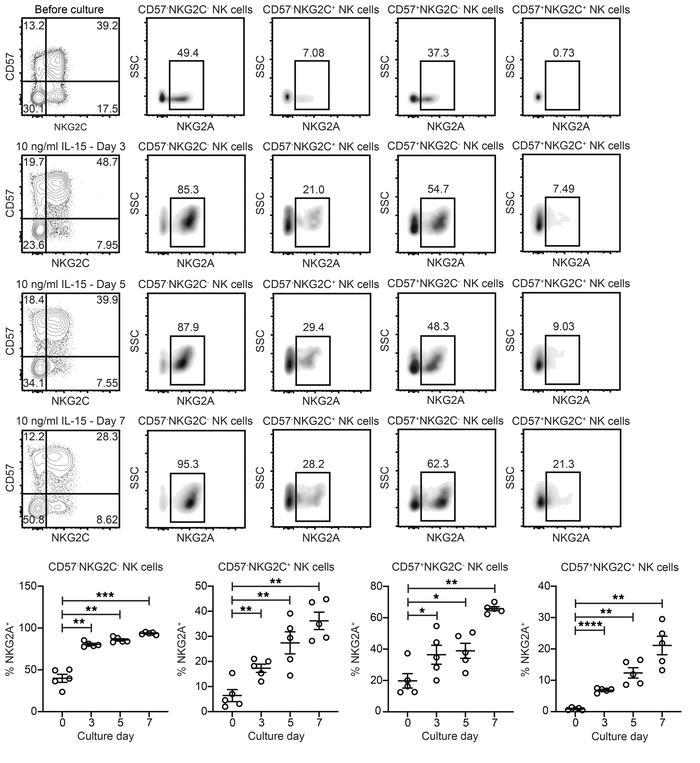
NKG2A is upregulated on the surface of NKG2C– and NKG2C+ NK cells during culture with IL-15.



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)