Abstract
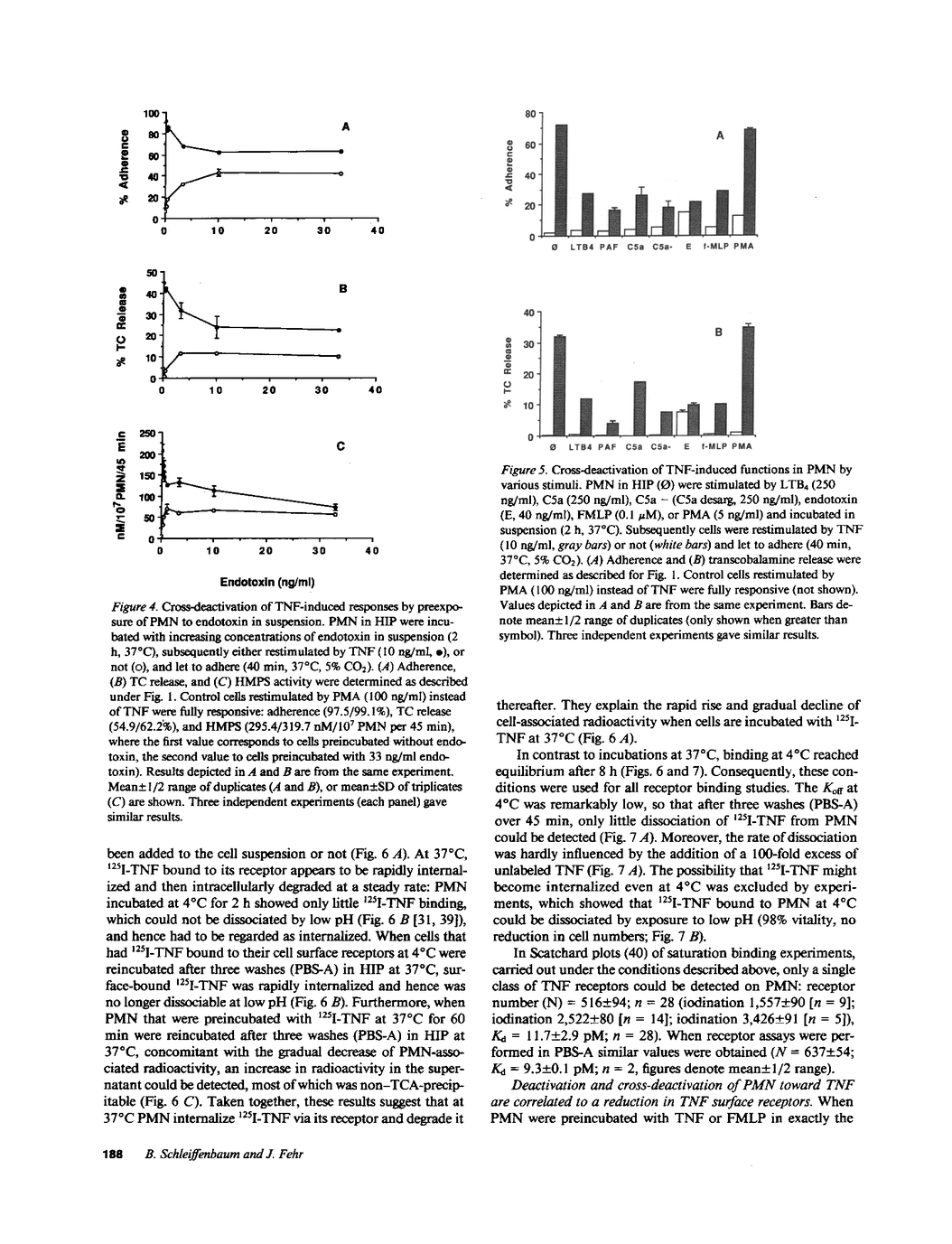
Despite numerous reports, the role of tumor necrosis factor (TNF) in polymorphonuclear leukocyte (PMN) function remains controversial. We found TNF to be a potent, pertussis toxin-independent stimulator of PMN adhesion (ED50 2.6 pM). TNF-stimulated PMN under adherent conditions released up to 65% of their transcobalamine content (ED50 3.9 pM) and increased their burst activity 10-fold (ED50 3.2 pM) as measured by the hexose monophosphate shunt, whereas PMN held in suspension hardly degranulated at all and only little burst activity was demonstrable. However, preincubation of PMN with TNF in suspension led to a decrease in cellular adhesiveness, degranulation, and burst activity in response to a secondary stimulus of TNF under adherent conditions, although cells remained fully responsive toward phorbol myristate acetate. A concomitant dose-dependent decline of TNF receptor numbers that correlated well with the inhibition of PMN function (r = 0.91) suggests receptor down-regulation as the mechanism of functional PMN deactivation. Remarkably, preincubation with other PMN stimuli such as N-formyl-methionyl-leucyl-phenylalanine, platelet-activating factor, leukotriene B4, complement component fragment 5a (C5a)/C5a (desarginated), and endotoxin also led to a reduction of TNF-specific PMN responses (cross-deactivation) from 35% (LTB4) to 90% (endotoxin), corresponding with the down-regulation of TNF receptors. Deactivation and receptor down-regulation are independent of pertussis toxin-sensitive G proteins and protein kinase C but seemed to depend on changes in calcium metabolism. Granulocyte hyporesponsiveness towards TNF in sepsis (with elevated blood levels of endotoxin and TNF) might be a mechanism of self-protection or, to the contrary, might impair a possibly central mode of host defense.
Authors
B Schleiffenbaum, J Fehr
Other pages:
| 184 | 185 | 186 | 187 | 188 | 189 | 190 | 191 | 192 | 193 | 194 | 195 |