Microbiology
Abstract
The fungal pathogen Candida albicans has a multilayered cell wall composed of an outer layer of proteins glycosylated with N- or O-linked mannosyl residues and an inner skeletal layer of β-glucans and chitin. We demonstrate that cytokine production by human mononuclear cells or murine macrophages was markedly reduced when stimulated by C. albicans mutants defective in mannosylation. Recognition of mannosyl residues was mediated by mannose receptor binding to N-linked mannosyl residues and by TLR4 binding to O-linked mannosyl residues. Residual cytokine production was mediated by recognition of β-glucan by the dectin-1/TLR2 receptor complex. C. albicans mutants with a cell wall defective in mannosyl residues were less virulent in experimental disseminated candidiasis and elicited reduced cytokine production in vivo. We concluded that recognition of C. albicans by monocytes/macrophages is mediated by 3 recognition systems of differing importance, each of which senses specific layers of the C. albicans cell wall.
Authors
Mihai G. Netea, Neil A.R. Gow, Carol A. Munro, Steven Bates, Claire Collins, Gerben Ferwerda, Richard P. Hobson, Gwyneth Bertram, H. Bleddyn Hughes, Trees Jansen, Liesbeth Jacobs, Ed T. Buurman, Karlijn Gijzen, David L. Williams, Ruurd Torensma, Alistair McKinnon, Donna M. MacCallum, Frank C. Odds, Jos W.M. Van der Meer, Alistair J.P. Brown, Bart Jan Kullberg
Abstract
Recent studies have shown that fine structural modifications of Mycobacterium tuberculosis cell envelope lipids mediate host cell immune activation during infection. One such alteration in lipid structure is cis-cyclopropane modification of the mycolic acids on trehalose dimycolate (TDM) mediated by proximal cyclopropane synthase of α mycolates (pcaA), a proinflammatory lipid modification during early infection. Here we examine the pathogenetic role and immunomodulatory function of mycolic acid cyclopropane stereochemistry by characterizing an M. tuberculosis cyclopropane–mycolic acid synthase 2 (cmaA2) null mutant (ΔcmaA2) that lacks trans-cyclopropanation of mycolic acids. Although titers of WT and ΔcmaA2 organisms were identical during mouse infection, ΔcmaA2 bacteria were hypervirulent while inducing larger granulomas than WT M. tuberculosis. The hypervirulence of the ΔcmaA2 strain depended on host TNF-α and IFN-γ. Loss of trans-cyclopropanation enhanced M. tuberculosis–induced macrophage inflammatory responses, a phenotype that was transferable with petroleum ether extractable lipids. Finally, purified TDM lacking trans-cyclopropane rings was 5-fold more potent in stimulating macrophages. These results establish cmaA2-dependent trans-cyclopropanation of TDM as a suppressor of M. tuberculosis–induced inflammation and virulence. In addition, cyclopropane stereochemistries on mycolic acids interact directly with host cells to both positively and negatively influence host innate immune activation.
Authors
Vivek Rao, Feng Gao, Bing Chen, William R. Jacobs, Michael S. Glickman
Abstract
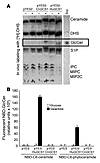
The pathogenic fungus Cryptococcus neoformans infects humans upon inhalation and causes the most common fungal meningoencephalitis in immunocompromised subjects worldwide. In the host, C. neoformans is found both intracellularly and extracellularly, but how these two components contribute to the development of the disease is largely unknown. Here we show that the glycosphingolipid glucosylceramide (GlcCer), which is present in C. neoformans, was essential for fungal growth in host extracellular environments, such as in alveolar spaces and in the bloodstream, which are characterized by a neutral/alkaline pH, but not in the host intracellular environment, such as in the phagolysosome of macrophages, which is characteristically acidic. Indeed, a C. neoformans mutant strain lacking GlcCer did not grow in vitro at a neutral/alkaline pH, yet it had no growth defect at an acidic pH. The mechanism by which GlcCer regulates alkali tolerance was by allowing the transition of C. neoformans through the cell cycle. This study establishes C. neoformans GlcCer as a key virulence factor of cryptococcal pathogenicity, with important implications for future development of new antifungal strategies.
Authors
Philipp C. Rittershaus, Talar B. Kechichian, Jeremy C. Allegood, Alfred H. Merrill, Mirko Hennig, Chiara Luberto, Maurizio Del Poeta
Abstract
Panton-Valentine leukocidin (PVL) is a pore-forming toxin secreted by Staphylococcus aureus that has recently been associated with necrotizing pneumonia. In the present study, we report that in vitro, PVL induces polymorphonuclear cell death by necrosis or by apoptosis, depending on the PVL concentration. PVL-induced apoptosis was associated with a rapid disruption of mitochondrial homeostasis and activation of caspase-9 and caspase-3, suggesting that PVL-induced apoptosis is preferentially mediated by the mitochondrial pathway. Polymorphonuclear cell exposure to PVL leads to mitochondrial localization of the toxin, whereas Bax, 1 of the 2 essential proapoptotic members of the Bcl-2 family, was still localized in the cytosol. Addition of PVL to isolated mitochondria induced the release of the apoptogenic proteins cytochrome c and Smac/DIABLO. Therefore, we suggest that PVL, which belongs to the pore-forming toxin family, could act at the mitochondrion level by creating pores in the mitochondrial outer membrane. Furthermore, LukS-PV, 1 of the 2 components of PVL, was detected in lung sections of patients with necrotizing pneumonia together with DNA fragmentation, suggesting that PVL induces apoptosis in vivo and thereby is directly involved in the pathophysiology of necrotizing pneumonia.
Authors
Anne-Laure Genestier, Marie-Cécile Michallet, Gilles Prévost, Gregory Bellot, Lara Chalabreysse, Simone Peyrol, Françoise Thivolet, Jerome Etienne, Gérard Lina, François M. Vallette, François Vandenesch, Laurent Genestier
Abstract
Molecular mimicry of Campylobacter jejuni lipo-oligosaccharides (LOS) with gangliosides in nervous tissue is considered to induce cross-reactive antibodies that lead to Guillain-Barré syndrome (GBS), an acute polyneuropathy. To determine whether specific bacterial genes are crucial for the biosynthesis of ganglioside-like structures and the induction of anti-ganglioside antibodies, we characterized the C. jejuni LOS biosynthesis gene locus in GBS-associated and control strains. We demonstrated that specific types of the LOS biosynthesis gene locus are associated with GBS and with the expression of ganglioside-mimicking structures. Campylobacter knockout mutants of 2 potential GBS marker genes, both involved in LOS sialylation, expressed truncated LOS structures without sialic acid, showed reduced reactivity with GBS patient serum, and failed to induce an anti-ganglioside antibody response in mice. We demonstrate, for the first time, to our knowledge, that specific bacterial genes are crucial for the induction of anti-ganglioside antibodies.
Authors
Peggy C.R. Godschalk, Astrid P. Heikema, Michel Gilbert, Tomoko Komagamine, C. Wim Ang, Jobine Glerum, Denis Brochu, Jianjun Li, Nobuhiro Yuki, Bart C. Jacobs, Alex van Belkum, Hubert P. Endtz
No posts were found with this tag.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)