Helminth co-infection exacerbates tuberculosis
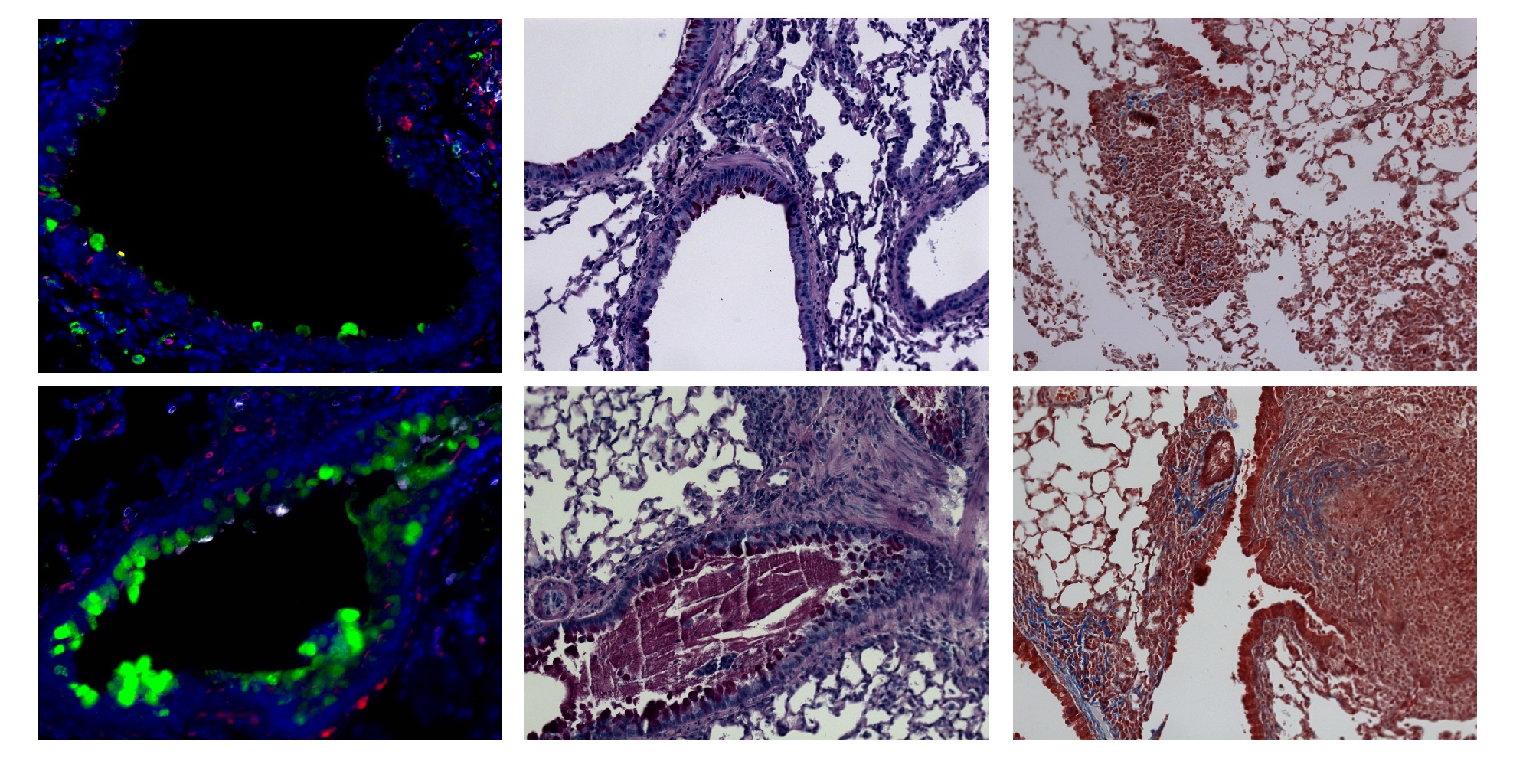
One third of the worlds’ population is infected with Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB). While the majority of Mtb-infected individuals are asymptomatic with the latent disease, millions of people present with active disease, which is one of the leading causes of death worldwide. In areas where parasitic helminth worms are endemic, the incidence of active TB is relatively high, suggesting that helminth infection exacerbates TB. Leticia Monin and colleagues at the Washington University School of Medicine used a mouse Mtb infection model to explore the relationship between helminth co-infection and Mtb manifestations. Co-infection with Schistosoma mansoni promoted fibrosis, increaed inflammation, and resulted in accumulation of arginase-1-expressing macrophages, which formed type 2 granulomas, in the lungs of in Mtb-infected mice. Treatment of co-infected animals with anti-helminth agents reduced S.mansoni burden, ameliorated inflammation, improved Th1-mediated anti-Mtb responses, and reduced disease severity. The authors infected a genetically diverse mouse cohort with Mtb alone and found that animals with severe disease also had elevated arginase-1 levels in the lungs. Evaluation of Mtb-infected patients revealed a correlation between serum levels of active arginase-1 and the extent of lung inflammation. Moreover, arginase-1 levels were highest in individulas that were also infected with helminthes. Together, these provide important insight into why TB disease rates are elevated in areas where helminthes are endemic. The accompanying image shows lung pathology of mice infected with Mtb (top row) or co-infected with Mtb and S. mansoni (bottom row). Note that co-infection increases the number of Muc5AC+ goblet cells (green, left panels), increases mucin and glycogen (PAS, middle panels), and increases collagen deposition within type 2 granulomas (Tricome, right panels).
Related articles
Abstract
Parasitic helminth worms, such as
Authors
Leticia Monin, Kristin L. Griffiths, Wing Y. Lam, Radha Gopal, Dongwan D. Kang, Mushtaq Ahmed, Anuradha Rajamanickam, Alfredo Cruz-Lagunas, Joaquín Zúñiga, Subash Babu, Jay K. Kolls, Makedonka Mitreva, Bruce A. Rosa, Rosalio Ramos-Payan, Thomas E. Morrison, Peter J. Murray, Javier Rangel-Moreno, Edward J. Pearce, Shabaana A. Khader



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

