Abstract
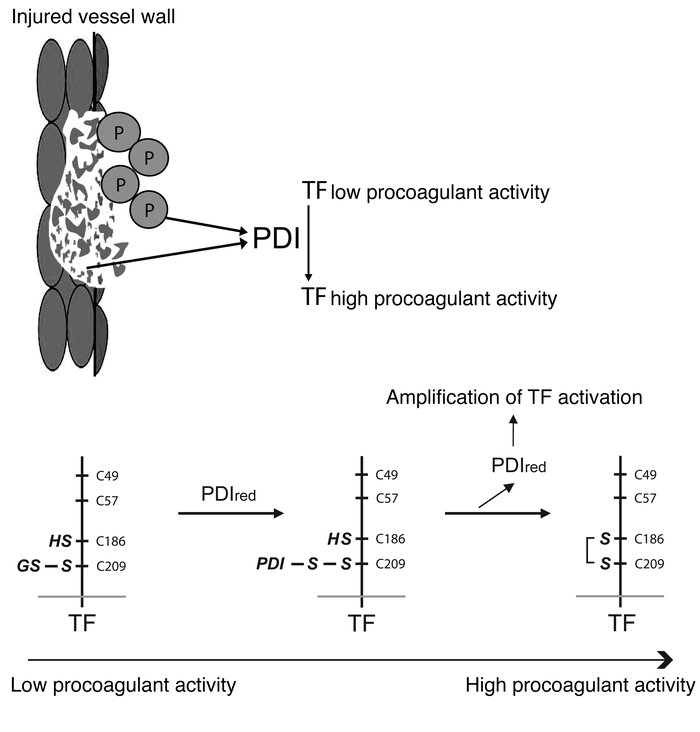
The activation of initiator protein tissue factor (TF) is likely to be a crucial step in the blood coagulation process, which leads to fibrin formation. The stimuli responsible for inducing TF activation are largely undefined. Here we show that the oxidoreductase protein disulfide isomerase (PDI) directly promotes TF-dependent fibrin production during thrombus formation in vivo. After endothelial denudation of mouse carotid arteries, PDI was released at the injury site from adherent platelets and disrupted vessel wall cells. Inhibition of PDI decreased TF-triggered fibrin formation in different in vivo murine models of thrombus formation, as determined by intravital fluorescence microscopy. PDI infusion increased — and, under conditions of decreased platelet adhesion, PDI inhibition reduced — fibrin generation at the injury site, indicating that PDI can directly initiate blood coagulation. In vitro, human platelet–secreted PDI contributed to the activation of cryptic TF on microvesicles (microparticles). Mass spectrometry analyses indicated that part of the extracellular cysteine 209 of TF was constitutively glutathionylated. Mixed disulfide formation contributed to maintaining TF in a state of low functionality. We propose that reduced PDI activates TF by isomerization of a mixed disulfide and a free thiol to an intramolecular disulfide. Our findings suggest that disulfide isomerases can act as injury response signals that trigger the activation of fibrin formation following vessel injury.
Authors
Christoph Reinhardt, Marie-Luise von Brühl, Davit Manukyan, Lenka Grahl, Michael Lorenz, Berid Altmann, Silke Dlugai, Sonja Hess, Ildiko Konrad, Lena Orschiedt, Nigel Mackman, Lloyd Ruddock, Steffen Massberg, Bernd Engelmann
Figure 9
Model for the activation of TF-dependent coagulation start by PDI.



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)