Citation Information: J Clin Invest. 2007;117(1):175-184. https://doi.org/10.1172/JCI29881.
Abstract
Adipose tissue macrophages (ATMs) infiltrate adipose tissue during obesity and contribute to insulin resistance. We hypothesized that macrophages migrating to adipose tissue upon high-fat feeding may differ from those that reside there under normal diet conditions. To this end, we found a novel F4/80+CD11c+ population of ATMs in adipose tissue of obese mice that was not seen in lean mice. ATMs from lean mice expressed many genes characteristic of M2 or “alternatively activated” macrophages, including Ym1, arginase 1, and Il10. Diet-induced obesity decreased expression of these genes in ATMs while increasing expression of genes such as those encoding TNF-α and iNOS that are characteristic of M1 or “classically activated” macrophages. Interestingly, ATMs from obese C-C motif chemokine receptor 2–KO (Ccr2-KO) mice express M2 markers at levels similar to those from lean mice. The antiinflammatory cytokine IL-10, which was overexpressed in ATMs from lean mice, protected adipocytes from TNF-α–induced insulin resistance. Thus, diet-induced obesity leads to a shift in the activation state of ATMs from an M2-polarized state in lean animals that may protect adipocytes from inflammation to an M1 proinflammatory state that contributes to insulin resistance.
Authors
Carey N. Lumeng, Jennifer L. Bodzin, Alan R. Saltiel
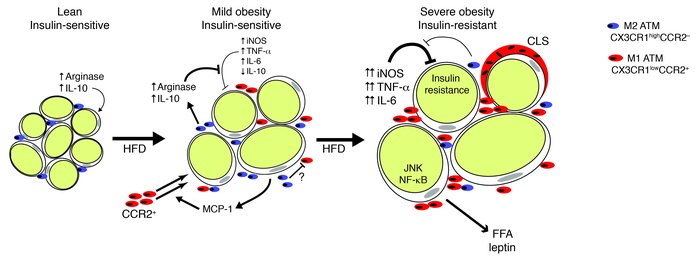
Figure 8
Proposed model for ATM polarization and its function in adipose tissue with progressive obesity.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)