Frances M. Ashcroft
Citation Information: J Clin Invest. 2005;115(8):2047-2058. https://doi.org/10.1172/JCI25495.
Frances M. Ashcroft
Published August 1, 2005
Citation Information: J Clin Invest. 2005;115(8):2047-2058. https://doi.org/10.1172/JCI25495.
Abstract
ATP-sensitive potassium (KATP) channels, so named because they are inhibited by intracellular ATP, play key physiological roles in many tissues. In pancreatic β cells, these channels regulate glucose-dependent insulin secretion and serve as the target for sulfonylurea drugs used to treat type 2 diabetes. This review focuses on insulin secretory disorders, such as congenital hyperinsulinemia and neonatal diabetes, that result from mutations in KATP channel genes. It also considers the extent to which defective regulation of KATP channel activity contributes to the etiology of type 2 diabetes.
Authors
Frances M. Ashcroft
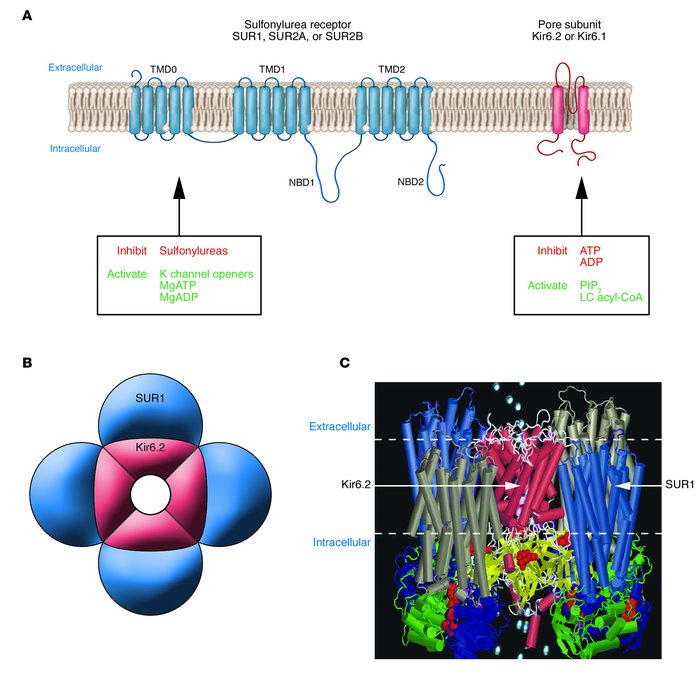
Figure 2
Options:
View larger image
(or click on image)
Download as PowerPoint
Molecular structure of the KATP channel. (A ) Schematic representation of the transmembrane topology of a single SURx (left) or Kir6.x (right) subunit. Mg-nucleotide binding/hydrolysis at the nucleotide-binding domains (NBD1, NBD2) of SUR stimulates channel activity. Sulfonylureas (stimulatory) and K channel openers (inhibitory) also bind to SUR1. Binding of ATP or ADP to Kir6.2 closes the pore, an effect that does not require Mg2+. Conversely, binding of phospholipids such as PIP2, or long-chain (LC) acyl-coAs, stimulates KATP channel activity and decreases its ATP sensitivity. (B ) Schematic representation of the octameric KATP channel complex viewed in cross section. Four Kir6.2 subunits come together to form the pore through which K+ ions move, and each is associated with a regulatory SURx subunit. (C ) Model of how SUR1 and Kir6.2 might assemble to form the KATP channel. The SUR model is described in ref. 115 and the Kir6.2 model in ref. 116 . The model illustrates that the channel complex contains 4 ATP-binding sites (on Kir6.2) and 8 Mg nucleotide–binding sites (on SUR1).



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)