Advertisement
Commentary
Open Access |  10.1172/JCI196707
10.1172/JCI196707
Unraveling endocannabinoid signaling disruption in a preclinical model of neurodevelopmental disorders
Nephi Stella
Departments of Pharmacology and Psychiatry and Behavioral Sciences, The University of Washington, Seattle, Washington, USA.
Address correspondence to: Nephi Stella, 1959 Pacific Ave. North, Seattle, Washington, 98195, USA. Phone: 206.221.5220; Email: nstella@uw.edu.
Find articles by Stella, N. in: PubMed | Google Scholar
Published September 2, 2025 - More info
J Clin Invest. 2025;135(17):e196707. https://doi.org/10.1172/JCI196707.
© 2025 Stella This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Related article:
Abstract
Protein phosphatase 2A (PP2A) is a serine/threonine phosphatase in the brain. Mutations in PPP2R1A, encoding the scaffolding subunit, are linked to intellectual disability, although the underlying mechanisms remain unclear. This study examined mice with heterozygous deletion of Ppp2r1a in forebrain excitatory neurons (NEX-het-conditional knockout [NEX-het-cKO]). These mice exhibited impaired spatial learning and memory, resembling Ppp2r1a-associated intellectual disability. Ppp2r1a haploinsufficiency also led to increased excitatory synaptic strength and reduced inhibitory synapse numbers on pyramidal neurons. The increased excitatory synaptic transmission was attributed to increased presynaptic release probability, likely due to reduced levels of 2-arachidonoyl glycerol (2-AG). This reduction in 2-AG was associated with increased transcription of monoacylglycerol lipase (MAGL), driven by destabilization of enhancer of zeste homolog 2 (EZH2) in NEX-het-cKO mice. Importantly, the MAGL inhibitor JZL184 effectively restored both synaptic and learning deficits. Our findings uncover an unexpected role of PPP2R1A in regulating endocannabinoid signaling, providing fresh molecular and synaptic insights into the mechanisms underlying intellectual disability.
Authors
Yirong Wang, Weicheng Duan, Hua Li, Zhiwei Tang, Ruyi Cai, Shangxuan Cai, Guanghao Deng, Liangpei Chen, Hongyan Luo, Liping Chen, Yulong Li, Jian-Zhi Wang, Bo Xiong, Man Jiang
-
Abstract
The search for transformative medicines has continuously uncovered select diseases associated with the disruption of the endocannabinoid (eCB) signaling system in the brain and emphasized the therapeutic value of small molecules that rescue this signaling system. In this issue of JCI, Wang et al. report that genetic disruption of PPP2R1A function in mouse forebrain, a preclinical mouse model of neurodevelopmental disorders, resulted in pronounced impairment of eCB signaling. Notably, small-molecule inhibitors of eCB inactivation rescued both eCB signaling and cognitive dysfunction in this model, providing a solid foundation to move such transformative therapeutic approaches based on targeting eCB signaling toward human clinical trial testing.
-
Sourcing therapeutic targets from the endocannabinoid system
Δ9-Tetrahydrocannabinol (THC) is the principal bioactive ingredient in the Cannabis plant, acting as a partial agonist at the endogenous cannabinoid receptor CB1R. Though the earliest therapeutic use of THC was likely for its analgesic properties (1), its therapeutic benefits have been explored for a range of CNS indications, including epilepsy, migraines, insomnia, multiple sclerosis, and Huntington’s disease (2). Its efficacy for these indications was systematically quite limited, and THC induces nontrivial neurological and systemic side effects, reducing its therapeutic value (3). Moreover, chronic use of high amounts of THC impacts brain development and function and is associated with increased incidence of mental health disorders, including cannabis use disorder and psychosis or schizophrenia (4).
Targeting the endocannabinoid (eCB) signaling system may be a way to bypass the therapeutic limitations of THC. The eCB signaling system plays a fundamental role in the control of synaptic transmission and plasticity as well as other physiological processes (5, 6). The two main eCBs, anandamide and 2-arachidonyl glycerol (2-AG), are signaling lipids that activate CB1R on neurons and astrocytes and CB2R on immune cells. 2-AG is abundantly present in the CNS, and its levels and activity at CB1R are tightly and differentially controlled by the enzymes MAGL and ABHD6 (7). Several preclinical studies have reported on the therapeutic value of MAGL inhibitors for the treatment of pain and inflammation, ischemic stroke, migraines, and anxiety-related disorders (8–10). Thus, targeting eCB inactivation represents a promising therapeutic approach with a unique mechanism of action, as it only boosts eCB signaling where it is physiologically engaged rather than broadly stimulating CB1R, as THC-based therapies do.
-
Connecting eCB impairment to synaptic and learning deficits
Children carrying genetic variants in the scaffolding subunit of the PP2A enzyme PPP2R1A exhibit symptoms typical of neurodevelopmental disorders, such as moderate-to-severe intellectual disability and developmental delay; but how PPP2R1A variants disrupt brain function has remained unclear. In this issue, Wang et al. show that the heterozygous deletion of the murine analog Ppp2r1a in forebrain excitatory neurons of mice (hereafter referred to as NEX-het-cKO mice) results in impairments in spatial learning and memory that model PPP2R1A-associated intellectual disability in humans (Figure 1A) (11). In NEX-het-cKO mice, heterozygous Ppp2r1a deletion increased excitatory synaptic strength. When RNA-Seq of the mouse forebrain tissue revealed a link between Ppp2r1a haploinsufficiency and upregulation of Magl, Wang and colleagues hypothesized that the eCB signaling system, and specifically 2-AG signaling, was involved in this change. Indeed, using fiber photometry measurements of a genetically encoded fluorescent eCB sensor, they observed disruption of 2-AG signaling in the NEX-het-cKO mouse forebrain. The increased excitatory synapse strength observed in NEX-het-cKO mice was driven by its increased presynaptic release probability, likely due to reduced levels of 2-AG that act at presynaptic CB1R, suggesting that normalizing 2-AG levels may ameliorate synaptic deficits in these mice. Accordingly, the MAGL inhibitor JZL184 restored both the synaptic and learning deficits in these mice (Figure 1B).
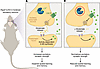 Figure 1
Figure 1Impaired eCB signaling underlies synaptic alterations and spatial learning and memory deficits in Ppp2r1a-deficient mice. The eCB 2-AG is produced by postsynaptic neurons and normally acts on presynaptic CB1R to reduce glutamate release. Wang et al. leveraged the recently developed GRABeCB2.0 sensor to detect the subcellular dynamics of 2-AG signaling via the fluorescence induced when extrasynaptic 2-AG binds to the sensor. (A) Heterozygotic genetic deletion of Ppp2r1a in mouse brain led to impaired 2-AG/CB1R signaling alongside increased excitatory synaptic strength and ensuing impairments in spatial learning in this model. (B) The sorted RNA-Seq data identified the upregulation of mRNA encoding for the presynaptic enzyme MAGL, which regulates tonic levels of 2-AG, in the Ppp2r1a-deficient mouse forebrain. Wang et al. showed that pharmacological inhibition of MAGL rescued 2-AG signaling, leading to normalization of excitatory synaptic strength and spatial learning and memory.
Although multiple studies have reported stimulus- and disease-associated changes in 2-AG levels in mouse brain, existing approaches were limited in cellular and temporal resolution, providing only an aggregate snapshot of its complex signaling dynamics. Here, Wang et al. have leveraged an innovative technology enabling measurement of rapid and transient changes in cell-specific production of eCBs: the GRABeCB sensors developed by the Li laboratory at Peking University (12). The recent development of fluorescence sensors based on GPCRs that are activated by lipid mediators has revolutionized our ability to study the subcellular dynamics of these hydrophobic signaling molecules (12). Such genetically encoded fluorescent sensors allow for real-time detection of changes in the levels of select endogenous signaling molecules (13). The GRABeCB2.0 sensor introduces a circularly permuted green fluorescent protein into the third intracellular loop of CB1R to elicit fluorescence upon 2-AG binding (14). Thus, GRABeCB2.0 detects changes in 2-AG levels with subsecond resolution in cells in culture, mouse brain slices, and in mouse brain during behavior (15–19). Using this approach was essential for Wang et al.’s discovery that the MAGL inhibitor, JZL184, increases 2-AG levels.
-
Limitations and next steps
While inhibiting MAGL activity represents a promising therapeutic approach, one must consider limitations associated with chronic MAGL inhibition, which include behavioral side effects and tolerance linked to CB1R desensitization (20–23). A possible explanation is that MAGL controls tonic 2-AG levels and ensuing elevated activity at CB1R leads to desensitization (7). The enzyme ABHD6, which controls only the stimulated production of 2-AG, could be considered as an alternative target, and the efficacy of its inhibitors does not appear to undergo tolerance (7). As the sorted RNA-Seq results presented in this study were suggestive of an increase in FAAH function (11), which constitutes another mechanism for controlling eCB signaling, it is also worth considering whether targeting FAAH would also rescue cognitive impairment in the Ppp2r1a-haploinsufficient mouse model. Furthermore, Wang et al.’s sorted RNA-Seq results identified many additional mRNAs that were greatly increased by Ppp2r1a haploinsufficiency, and analysis of these mRNAs by clustering GeneSets might reveal additional signaling pathways disrupted in this model, highlighting additional potential therapeutic targets. An exciting new line of research will be to identify the molecular mechanism that links heterozygote deletion of PPP2R1A to changes in the expression of MAGL that control 2-AG signaling.
The extent to which PPP2R1A regulates eCB signaling in this mouse model is quite pronounced, providing a solid foundation for future biological and mechanistic studies as well as follow-up preclinical investigations. These endeavors will foster the development of potentially transformative therapeutic approaches for the treatment of neurodevelopmental disorders as they will act via unexplored mechanisms of action. Although the first steps down a new line of clinical investigation are always uncertain, this study represents a strong lead for developing eCB-based therapies with the potential to have tremendous effect on patients.
-
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Copyright: © 2025, Stella et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(17):e196707. https://doi.org/10.1172/JCI196707.
See the related article at Ppp2r1a haploinsufficiency increases excitatory synaptic transmission and decreases spatial learning by impairing endocannabinoid signaling.
-
References
- Singh S, et al. Analgesic properties of next generation modulators of endocannabinoid signaling: leveraging modern tools for the development of novel therapeutics. J Pharmacol Exp Ther. 2024;391(12):162–173.
- Stella N. THC and CBD: similarities and differences between siblings. Neuron. 2023;111(3):302–327.
- Hollister LE. Tetrahydrocannabinol isomers and homologues: contrasted effects of smoking. Nature. 1970;227(5261):968–969.
- Mennis J, et al. Young adult cannabis use disorder treatment admissions declined as past month cannabis use increased in the U.S.: An analysis of states by year, 2008-2017. Addict Behav. 2021;123:107049.
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14(9):923–930.
- Bloomfield MA, et al. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature. 2016;539(7629):369–377.
- Cao JK, et al. ABHD6: Its place in endocannabinoid signaling and beyond. Trends Pharmacol Sci. 2019;40(4):267–277.
- Ren S-y, et al. Potential application of endocannabinoid system agents in neuropsychiatric and neurodegenerative diseases-focusing on FAAH/MAGL inhibitors. Acta Pharmacol Sin. 2020;41(10):1263–1271.
- Bononi G, et al. An updated patent review of monoacylglycerol lipase (MAGL) inhibitors (2018-present). Expert Opin Ther Pat. 2021;31(2):153–168.
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11(1):51–62.View this article via: PubMed Google Scholar
- Wang Y, et al. Ppp2r1a haploinsufficiency increases excitatory synaptic transmission and decreases spatial learning by impairing endocannabinoid signaling. J Clin Invest. 2025;135(17):e185602.
- Dong C, et al. Fluorescence imaging of neural activity, neurochemical dynamics, and drug-specific receptor conformation with genetically encoded sensors. Annu Rev Neurosci. 2022;45(1):273–294.
- Labouesse MA, Patriarchi T. A versatile GPCR toolkit to track in vivo neuromodulation: not a one-size-fits-all sensor. Neuropsychopharmacology. 2021;46(12):2043–2047.
- Ravotto L, et al. A bright and colorful future for g-protein coupled receptor sensors. Front Cell Neurosci. 2020;14:67.
- Dong A, et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nat Biotechnol. 2022;40(5):787–798.
- Farrell JS, et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron. 2021;109(15):2398–2403.
- Liput DJ, et al. 2-Arachidonoylglycerol mobilization following brief synaptic stimulation in the dorsal lateral striatum requires glutamatergic and cholinergic neurotransmission. Neuropharmacology. 2022;205:108916.
- Liu Z, et al. Deficiency in endocannabinoid synthase DAGLB contributes to Parkinson’s disease and dopaminergic neuron dysfunction. Nat Commun. 2022;13(1):3490.
- Singh S, et al. Pharmacological characterization of the endocannabinoid sensor GRABeCB2.0. Cannabis Cannabinoid Res. 2024;9(5):1250–1266.
- Manterola A, et al. Re-examining the potential of targeting ABHD6 in multiple sclerosis: efficacy of systemic and peripherally restricted inhibitors in experimental autoimmune encephalomyelitis. Neuropharmacology. 2018;141:181–191.
- Schlosburg JE, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13(9):1113–1119.
- Owens RA, et al. Inhibition of the endocannabinoid-regulating enzyme monoacylglycerol lipase elicits a CB1 receptor-mediated discriminative stimulus in mice. Neuropharmacology. 2017;125:80–86.
- Guindon J, et al. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br J Pharmacol. 2011;163(7):1464–1478.
-
Version history
- Version 1 (September 2, 2025): Electronic publication



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

