Advertisement
Review
Open Access |  10.1172/JCI194312
10.1172/JCI194312
Particulate matter air pollution: effects on the respiratory system
Robert B. Hamanaka and Gökhan M. Mutlu
Department of Medicine, Section of Pulmonary and Critical Care Medicine, The University of Chicago, Chicago, Illinois, USA.
Address correspondence to: Gökhan M. Mutlu, The University of Chicago, Section of Pulmonary and Critical Care Medicine, 5841 S. Maryland Avenue, MC6026, Chicago, Illinois 60637, USA. Phone: 773.702.1002; Email: gmutlu@uchicago.edu.
Find articles by Hamanaka, R. in: PubMed | Google Scholar
Department of Medicine, Section of Pulmonary and Critical Care Medicine, The University of Chicago, Chicago, Illinois, USA.
Address correspondence to: Gökhan M. Mutlu, The University of Chicago, Section of Pulmonary and Critical Care Medicine, 5841 S. Maryland Avenue, MC6026, Chicago, Illinois 60637, USA. Phone: 773.702.1002; Email: gmutlu@uchicago.edu.
Find articles by Mutlu, G. in: PubMed | Google Scholar
Published September 2, 2025 - More info
J Clin Invest. 2025;135(17):e194312. https://doi.org/10.1172/JCI194312.
© 2025 Hamanaka et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Air pollution comprises a complex mixture of gaseous and particulate components. Particulate matter (PM) air pollution is associated with 4.7 million premature deaths per year. Among modifiable risk factors, air pollution exposure contributes to 8% of disability adjusted life years and ranks above factors such as high blood pressure, smoking, and high fasting plasma glucose. As the site of entry, exposure to PM air pollution causes respiratory symptoms and is a significant cause of respiratory morbidity and mortality. In this Review, we discuss the studies that link air pollution exposure with respiratory diseases. We review the epidemiological evidence linking PM exposure and lung diseases including asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, pneumonia, acute respiratory distress syndrome, and lung cancer. We also provide an overview of current knowledge about the mechanisms by which PM exerts its biological effects leading to adverse health effects in the respiratory system.
Air pollution is composed of a complex mixture of particulate and gaseous components. Toxicological and epidemiological study of air pollutants began in earnest in the mid-20th century after industrial smog events, including the Great Smog of London of 1952, caused acute rises in hospitalizations and deaths. These studies have consistently shown that ambient air pollution is the largest environmental health risk factor, contributing to as many as 4.7 million premature deaths per year (1). Among modifiable risk factors, air pollution exposure contributes to 8% of disability adjusted life years, ranking above factors such as high blood pressure, smoking, and high fasting plasma glucose (2). Most of the excess deaths linked to air pollution are due to acute ischemic/thrombotic cardiovascular events (3); however, chronic air pollution exposure is also a significant cause of respiratory morbidity and mortality. In this Review, we discuss the studies that link air pollution exposure with respiratory diseases in humans.
-
Components of air pollution
The components of air pollution vary greatly depending on the source of production, emission rate, and weather conditions. Gaseous components of air pollution include sulfur dioxide (SO2), nitrogen dioxide (NO2), nitric oxide (NO), ozone (O3), and carbon monoxide (CO) (4, 5). The particulate component of air pollution consists of carbon-based particles onto which various metals and organic chemicals are adsorbed. Common components of particulate matter (PM) are elemental carbon and organic carbon molecules, such as polyaromatic hydrocarbons (PAHs), nitrates, sulfates, sodium ion, and silicon as well as metals, including transition metals (e.g., Cd, Co, Cr, Fe, Ni, vanadium [V]) (6, 7) (Figure 1). PM is categorized based on particle size. Coarse particles with a diameter of less than 10 μm (PM10) are derived from both natural and industrial sources. These particles do not generally penetrate beyond the upper airway. Fine particles have a diameter of less than 2.5 μm (PM2.5), and ultrafine particles (nanoparticles) are those that are less than 0.1 μm diameter (PM0.1). They are produced by combustion of fossil fuels and penetrate into the small airways and alveoli (8, 9). This makes PM2.5 and PM0.1 greater threats to respiratory health than PM10.
 Figure 1
Figure 1Particulate matter size and components. (A) Images of urban particulate matter (PM) generated by scanning electron microscopy. Original magnification, ×2,000 (left), ×10,000 (center), and ×50,000 (right). (B) Relative sizes of PM10 (coarse), PM2.5 (fine), and PM0.1 (ultrafine or nanoparticles) in comparison to alveolar macrophage and influenza A virus (IAV). (C) Common components of PM. Other category includes metals (Al, K, Ca), transition metals (Fe, Zn, Cd, Ti, Ag, Cu, Mn, Au, Mg, Hg, Cr, Zr, Ni, V, and Co), nonmetals, halogens, and lanthanides (7, 272).
PM and NO2 (along with O3, which is a secondary pollutant) are the main industrial and traffic-related air pollutants measured in the industrialized world. SO2 is produced from combustion of high sulfur-containing fuels such as coal, and its levels have decreased in most of the world (6, 10). Indoor production of air pollutants through cooking and heating are also major sources of exposure-related health effects, particularly in the developing world, where burning of biomass is a major source of energy (11). Due to the coproduction of air pollutants, it is often difficult to determine the independent contribution of each pollutant with health effects; however, levels of PM2.5 have consistently been correlated negatively with cardiovascular and respiratory outcomes. Under the Clean Air Act, the US Environmental Protection Agency regulates PM2.5 and PM10 levels to establish National Ambient Air Quality Standards to protect public health and the environment (12) (Table 1).
-
Epidemiology
Time-series studies. The Great Smog of London directly contributed to an estimated 12,000 deaths, with tens of thousands more suffering adverse health effects afterward (13, 14). Similar observations linked with industrial smog events in Europe and the United States also provided evidence for the adverse effects of air pollution exposure on health; however, it was not until the late 20th century that a greater understanding of confounding factors such as weather, temperature, and lag effects as well as advances in statistical analysis allowed for epidemiological associations between air pollution and health outcomes to become apparent.
Large, multicity epidemiological studies, including the National Morbidity, Mortality, and Air Pollution Study (NMMAPS) and Air Pollution and Health: A European Approach (APHEA) studies, showed that increases in PM and other pollutants were associated with significant increases in all-cause mortality (15–19). Increased hospitalizations from cardiovascular and respiratory events were also associated with increases in PM levels (20, 21).
The recent Multi-City Multi-Country (MCC) study collected data from over 600 cities, mainly in North America, Europe, and Eastern Asia, and reported similar findings to those of the NMMAPS and APHEA studies, associating PM2.5 concentrations with total, cardiovascular, and respiratory mortality (22, 23). This study showed that associations between mortality and PM concentrations were strongest in locations with lower average annual PM concentrations. This finding is consistent with previous studies that showed a biphasic relationship between PM and mortality in which a steep concentration-response relationship is observed at lower PM concentrations, while the curve flattened at higher concentrations (24–26). This flattening of the curve is interpreted to be due to the higher basal PM levels seen in more polluted cities; however, other factors such as younger populations in developing countries may also play a role. The MCC study also confirmed the findings of the NMMAPS and APHEA studies that there is no threshold value of PM pollution below which positive associations are not detectable between exposure and deaths.
Cohort studies. The repeated finding that there exists no threshold below which air pollution levels are considered “safe” has led to three recent long-term studies in areas of low ambient pollution in the United States (US Medicare Study) (27), Canada (Mortality-Air Pollution Associations in Low-Exposure Environments [MAPLE] Study) (28), and Europe (Effects of Low-Level Air Pollution: A Study in Europe [ELAPSE Study]) (29), which use large administrative cohorts and advanced exposure assessment techniques to determine the effects of air pollution on health below current air quality standards. All three studies found associations between PM2.5 levels and mortality down to the lowest observed PM2.5 level of 4 μg/m3 (30). Significant correlations between NO2 and PM2.5 levels were observed with respiratory-related mortality at levels below the current WHO guideline values for both pollutants (29, 31). The findings from these new large studies mostly corroborate the results from older studies using smaller cohorts, including the Harvard Six Cities Study, the American Cancer Association Cancer Prevention Study, and European Study of Cohorts for Air Pollution Effects (ESCAPE) (32–34).
Interventional and other studies. The implementation of the Clean Air Act in 1970 resulted in a progressive decline in air pollution levels in the United States. While the decline has occurred gradually over an extended period of time, extended analysis of the NMMAPS and Harvard Six Cities Study revealed that reductions in PM2.5 levels contributed to significant increases in life expectancy (35–37). More drastic changes in air pollution occurred in China after the implementation of the Air Pollution Prevention and Control Action Plan (APPCAP) in 2013. Between 2013 and 2017, annual average PM2.5 concentrations decreased by one-third, leading to an estimated 47,000 fewer deaths in the 47 cities studied (38).
Recent years have offered several other natural experiments with which the association of abrupt changes in air pollution with health effects can be measured. Before and during the 2008 Olympic Games, Chinese government implemented emission control policies in Beijing and the surrounding area that reduced particulate and gaseous pollutants, including an average 31% decrease in PM2.5 (39). This was associated with reductions in pulmonary and systemic markers of inflammation in study participants (40–42) as well as reductions in emergency room visits for cardiovascular and asthma-related events and a decrease in cardiovascular mortality (42–47).
The COVID-19 pandemic led to wide-spread societal shutdowns to limit transmission of the SARS-CoV-2 virus. These shutdowns led to a 31% reduction in PM2.5 levels measured across 34 countries during the shutdown periods (48). Early studies have shown that during the time of these policies, significant reductions in non–COVID-19–associated mortality were detected, to which reductions in traffic accidents and exposure to air pollution are proposed as causal (49, 50). Air pollution monitoring and epidemiological modeling allowed for the calculation of the avoided mortality due to improvements in air quality. Reductions in premature mortality were particularly strong in China, which had the most stringent COVID-19 containment policies (51–53).
Exposure to wildfire smoke is a growing public health concern, with pollution due to wildfires increasingly influencing average annual PM2.5 concentrations in the United States (54). Health effects of wildfire smoke on cardiovascular and respiratory-related deaths are similar to those seen with industry- and traffic-related pollution (55, 56). Increases in hospital visits and admissions for cardiovascular and respiratory diseases have been seen in Southern California during increased wildfire activity (57–59). Wildfire smoke exposure in California is estimated to have caused over 50,000 premature deaths between 2008 and 2018 (60). Increased asthma- and cardiopulmonary-related hospital visits were also found after wildfire smoke from Quebec spread throughout the northeastern United States in June 2023 (61–63).
The destruction of the New York World Trade Center on September 11, 2001, resulted in an atmospheric dust plume containing thousands of tons of PM from pulverized building materials and combustion products of the fires (64). Many first responders experienced persistent cough accompanied by respiratory symptoms that required medical leave for at least four weeks (65, 66). Extended analysis of lung function from first responders showed an acute decline in lung function (measured by forced expiratory volume in 1 second [FEV1]) that did not recover even after years of follow-up (67, 68). Asthma, persistent airway hyperreactivity, obstructive airway disease, and interstitial lung disease have been associated with exposure to the World Trade Center dust (69–72).
-
Exposure to air pollution and respiratory disease
Strong associations exist between air pollution exposure and total mortality as well as cardiovascular and respiratory mortality. The MCC study showed that each 10 μg/m3 increase in PM concentration was associated with a 0.47% (95% CI, 0.35%–0.58%) rise in respiratory mortality (22). Using International Classification of Diseases (ICD) codes, the ELAPSE project studied four outcomes: nonaccidental, cardiovascular, nonmalignant respiratory, and lung cancer mortality. Significant associations were found between levels of PM2.5 and all four mortality outcomes, with lung cancer mortality showing the strongest relationship (29). Follow-up studies showed that PM2.5 levels significantly correlated with incidence of asthma, COPD, and lung cancer (73–75). The US Medicare Study, which did not stratify mortality based on cause, found that all-cause and respiratory hospital admissions were significantly correlated with exposure to PM2.5 (76). Below we discuss the epidemiologic and experimental evidence for the associations of air pollution exposure with lung diseases.
Asthma. Early experiments exposing humans to SO2 showed that patients with asthma have greater sensitivity to pollutant exposure than individuals acting as controls (77–79). Subsequent studies have consistently shown that the average local levels of air pollutants, including PM2.5, strongly correlate with severity of asthma symptoms and medication usage (80–83), emergency department visits (84–87), and hospitalizations (88–90). A classic example of these observations centers around a labor dispute that led to the closing of a steel mill in Utah Valley for one year over 1986 and 1987. This led to an over 50% reduction in the mean daily high PM10 levels. Inpatient admissions to local hospitals for children with bronchitis or asthma similarly halved during the period that the mill was closed (91, 92).
It is also increasingly accepted that air pollution exposure causes new-onset asthma, with evidence being particularly strong for childhood exposure. Birth cohort studies that track residential air pollution exposure have consistently shown that local air pollution, particularly PM2.5 and NO2 levels, correlate with development of childhood asthma (93–96). The Southern California Children’s Health Study found that children who live in high pollution areas, particularly near major roadways, show reduced lung function (as measured by FEV1, forced vital capacity [FVC], and maximum mid-expiratory flow rate [MMEF]) and higher incidence of asthma compared with children from less-polluted areas (97–101). Individuals who moved during the course of the study to areas of lower pollution showed increased rate of MMEF growth compared with participants who did not move (102). As air quality increased during the course of the study, improvements in lung function measurements and reduced incidence of asthma were noted (103, 104).
COPD. As with asthma, patients with COPD suffer increases in disease-related health events after increases in local air pollution. Daily gaseous and particulate pollutant levels correlate with reduced respiratory function (FEV1 and FVC) and recorded respiratory symptoms and medication usage by patients with COPD (105–108). Emergency room visits, hospital admissions, and mortality due to COPD are positively correlated with elevations in daily particulate and gaseous pollutants (109–114). Patients with COPD are also more susceptible to mortality after local increases in PM2.5 than the general population (115).
While tobacco smoking is the greatest risk factor for COPD, increasing evidence suggests that air pollution exposure is also a risk factor. Two recent large cross-sectional studies have shown that local PM2.5 levels correlate significantly with the incidence of COPD (116, 117). A large longitudinal study showed that each 5 μg/m3 increase in 2-year average PM2.5 level was associated with a decrease of 1.18% in FVC and 1.46% decrease in FEV1. Compared with the participants exposed to the lowest PM2.5 levels, patients with the highest exposure had a hazard ratio of 1.39 (95% CI, 1.16–1.46) for COPD development (118).
Pneumonia. Correlations of air pollution exposure with respiratory infections have been observed for nearly 100 years (119). Recent estimates suggest that every 10 μg/m3 increase in PM2.5 is associated with a 5.4% increase in respiratory tract infections in the Medicare population (120). Children also show heightened sensitivity to PM air pollution, which significantly correlates with pneumonia incidence (121).
Influenza infection is the best studied cause of pollution-associated pneumonia. Multiple studies have shown correlations between PM2.5 levels as well as wildfire smoke and incidence of influenza infection (122–125). Correlations of PM2.5 with respiratory syncytial virus and SARS-CoV2 infections have also been reported (125–128). While bacterial pneumonias are not as commonly diagnosed as viral pneumonias, mycoplasma pneumonia as well as tuberculosis have also been positively correlated with exposure to PM2.5 (129, 130), suggesting that the effects of air pollution exposure on pneumonia are not limited to viral infections.
Acute respiratory distress syndrome. Although acute respiratory distress syndrome (ARDS) has heterogeneous causes, including pneumonia, sepsis, trauma, or aspiration, downstream common pathways include inflammatory responses, immune infiltration into the lung, increased endothelial and epithelial permeability, and dysregulated coagulation (131, 132). Increasing findings suggest that air pollution exposure increases the likelihood of developing ARDS. A cohort study of critically ill patients stratified by air pollution exposures at their residences showed that the 3-year average exposure to PM2.5, is significantly associated with development of ARDS (133). Exposure to PM2.5 was also linked with an increased 90-day mortality rate from ARDS (134).
SARS-CoV-2 infection during the COVID-19 pandemic was a significant cause of ARDS. Several studies have shown correlations between residential exposure to air pollution and severity of SARS-CoV-2 infection and mortality (135–138). Annual PM2.5 exposure most closely correlated with COVID-19 hospitalization and death. Similar findings were found when exposure to wildfire smoke was correlated with COVID-19 cases and deaths (139).
Pulmonary fibrosis. Compared with other lung diseases, the link between pulmonary fibrosis (PF) and air pollution exposure has only recently been uncovered. The first linkages between PF and air pollution were associations between acute exacerbations and increases in NO2 and O3 levels (140). Patients with PF have since been shown to exhibit lower lung function (FVC) (141), increased rate of FVC decline (142), and increased rates of mortality associated with exposure level to PM2.5 (143). Although these were relatively small studies, a recent larger prospective cohort study showed an inverse correlation between PM2.5 exposure and transplant-free survival. Patients with higher PM2.5 exposure also had a lower baseline FVC and more rapid FVC decline (144). Another large retrospective study showed that daily hospital admissions based on ICD codes for PF correlated with PM2.5 levels, both on the day of admission and average levels in the preceding 4 days (145). There was no correlation when analysis was performed on average pollutant levels for the preceding 30 days, providing evidence of the acute impairment caused by pollution in patients with PF.
More recently, evidence has emerged that incidence of PF also correlates with chronic exposure to air pollution. Each interquartile range increase in PM2.5 at residential addresses of UK Biobank participants was found to correlate with a hazard ratio of 1.09 (95% CI, 1.02–1.17) for incidence of PF (146). High attenuation area (HAA) and interstitial lung abnormalities (ILA) are chest tomography-based measures used to identify subclinical forms of interstitial lung diseases and PF. Exposure to PM correlated with the progression of HAA in a prospective cohort enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) (147). Elemental carbon (a component of PM) exposure correlated with both ILA incidence and progression in participants of the Framingham Heart Study (148). These findings suggest that air pollution exposure may promote the earliest stages of subclinical interstitial and fibrotic lung diseases.
Lung cancer. Lung cancer is leading cause of cancer-associated death, and while smoking is the primary risk factor for lung cancer, one-third of lung cancer occurs in nonsmokers, making lung cancer in nonsmokers the fifth leading cause of cancer-associated death (149). The connection between air pollution exposure and lung cancer has been acknowledged since the 1950s (150), and large epidemiological studies have correlated exposure to multiple pollutants, particularly PM2.5, with both lung cancer incidence (75, 151, 152) and mortality (29, 153, 154).
Air pollution exposure has also been shown to decrease survival after lung cancer diagnosis. After adjustment for demographic factors, tumor characteristics at diagnosis, and treatment, patients with early-stage tumors living with low PM2.5 exposure (<10 μg/m3) had a median survival of 5.7 years compared with 2.4 years for patients with high PM2.5 exposure (>16 μg/m3) (155). Survival after lobectomy was also shown to be reduced by high exposure to PM2.5 (156).
Lung transplant. As would be expected from the negative effects on pollution on PF and COPD outcomes, annual average PM2.5 levels at the residential addresses of lung transplant candidates are associated with increased rates of removal from transplant waiting lists either due to mortality or from clinical deterioration of the patient (157). Air pollution exposure is also correlated with negative outcomes after lung transplantation. Posttransplant development of bronchiolitis obliterans or chronic lung allograft dysfunctions are associated with residential traffic density and proximity to a major roadway (158–160). Transplant recipients with higher PM2.5 exposure exhibited reduced FVC and FEV1 and increased rates of graft failure or death (161–163).
-
Biologic mechanisms
While the epidemiological evidence for the effect of PM exposure on lung health is increasingly clear, insight into the mechanisms by which PM exerts its harmful effects is also increasingly understood (Figure 2). Experimental systems have been designed to allow study of both in vivo and in vitro effects of exposure to air pollution particulates. Ambient fine particle concentrators enrich ambient PM2.5 from the local air, allowing for inhalation of concentrated ambient particles (CAPs) by humans or animal test subjects (164). Particles can also be collected on filters and used to treat cultured cells in vitro or can be suspended and instilled into the lungs of test animals when inhalation systems are unavailable. Standard reference materials (SRMs), such as SRM1649 Urban Dust and SRM2786, which are commercially available, and certified by the National Institute of Standards and Technology, allow for in vitro exposures of particles that are uniform across laboratories (165). Below, we discuss the current understanding of the mechanisms by which PM exposure leads to lung disease.
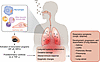 Figure 2
Figure 2Mechanisms by which PM air pollution affects the respiratory system. Inhaled PM induces an inflammatory response in the lung. PM acts on the cells of the lung, including airway and alveolar epithelial cells and macrophages, causing mitochondrial ROS-dependent transcriptional responses, including NF-κB and NRF2 activation. Oxidative stress promotes production of proinflammatory cytokines and oxidative damage. These PM-induced changes cause lung and systemic inflammation (due to spillover of cytokines into the circulation), altered immune response, and epigenetic changes. Clinically, PM-induced effects are exhibited through the development of respiratory symptoms such as cough and dyspnea in healthy individuals. PM exposure is associated with the development, progression, and exacerbation of lung diseases, including asthma, COPD, pulmonary fibrosis, pneumonia, ARDS, and lung cancer.
Oxidative stress and inflammation. It is widely understood that air pollution exerts many of its biologic effects by causing oxidative stress, which promotes subsequent inflammatory responses. Metals contained in PM are capable of redox cycling, and other chemicals adsorbed onto particles, including PAHs, can generate redox-active quinones. NO2 and O3 are reactive and lead to free radical accumulation (166, 167). Increases in ambient PM2.5 or controlled exposure to CAPs are associated with increased levels of DNA and lipid oxidation products in blood, urine, and breath condensate (40, 168, 169). These markers correlate with inflammatory markers such as IL-1β, IL-6, GM-CSF, TNF-α, fibrinogen, and C-reactive protein (170–173).
At the cellular level, in vitro exposure of lung cells, including nasal, airway, and lung epithelial cells, macrophages, and endothelial cells to particles isolated either from ambient air or from diesel exhaust leads to elevations in cellular reactive oxygen species (ROS) levels (174–182). This stress environment activates transcriptional programs, including those regulated by the oxidative stress–responsive transcription factor NRF2 and the proinflammatory transcription factor NF-κB (178, 183, 184). Oxidative stress is required for the biologic effects of PM exposure, as genetic inhibition of oxidant production or treatment of cells with antioxidants is sufficient to inhibit inflammatory cytokine production and proapoptotic signaling (174, 175, 185–188). NRF2-deficient mice exhibited greater lung inflammation after a 24-week inhalation exposure to CAPs, demonstrating the key role that oxidative stress plays in air pollution–induced inflammation (189).
The oxidative and inflammatory environment caused by exposure to air pollution likely plays an important role in the development of inflammatory airway diseases. Markers of oxidative stress are increased in the blood, urine, and breath condensate of patients with asthma and COPD (190, 191). Air pollution–linked cytokines such as IL-1β, IL-6, and TNF-α are highly elevated in the lungs of patients with COPD and play roles in modifying asthma phenotypes (192, 193). Furthermore, both diseases are associated with gene polymorphisms related to oxidative stress phenotypes, suggesting that an individual’s sensitivity to oxidative stress may regulate susceptibility to airway disease (194, 195).
Increased lung inflammation may also be an important cancer-promoting mechanism of air pollution. In a recent study, tumor formation in EGFR- or KRAS-mutant mice was accelerated by intratracheal instillation of exposure to PM (SRM2786) for 3 weeks (196). Despite this difference in tumor burden, there was no significant increase in mutational burden in the PM-exposed mice. The investigators demonstrated that production of IL-1β by lung macrophages promotes expansion of mutant cells and that inhibition of IL-1β using neutralizing antibodies prevented PM-promoted tumor formation. In nonmalignant human lung tissue from two separate clinical cohorts, EGFR and KRAS mutations were present in 18% and 53% of samples, respectively (196). Thus, these findings suggest that the primary mechanism by which air pollution exposure promotes cancer is through inflammatory effects on cells with preexisting mutations. Indeed, a study of UK Biobank participants in which single nucleotide polymorphism data were analyzed alongside air pollution exposure data showed an additive interaction between genetic risk factors and air pollution exposure (197). High air pollution exposure, particularly to PM2.5, increased the risk of developing lung cancer in all participants, with patients with high genetic risk scores and high pollution exposure at the greatest risk for lung cancer.
Mitochondrial dysfunction. While air pollution gases and PM-adsorbed chemicals and metals can generate free radicals, increasing evidence points to changes in mitochondrial function and mitochondrial production of ROS playing key roles in the response to air pollution. Air pollution particles have been shown to accumulate in the mitochondria of cultured airway epithelial and macrophages, leading to changes in mitochondrial morphology and increased oxidative stress (179, 198–203). Lung cells, including alveolar macrophages (AMs) and epithelial cells, that have been genetically engineered to be deficient in mitochondrial ROS production, or treated with mitochondria-targeted antioxidants or electron transport chain inhibitors, exhibit attenuated inflammatory responses to particles in culture (175, 186, 203, 204).
Similar to air pollution particulates, exposure of cells to cigarette smoke (CS) extract display altered mitochondrial function and increased ROS production (205–207). These mitochondrial changes are also observed in airway epithelial cells from CS-exposed mice and from patients with COPD (207–209). Alterations in mitochondrial turnover may play a role in linking mitochondrial dysfunction with lung phenotypes, as the mitophagy regulator PINK1 was found to be highly expressed in lung tissue of patients with COPD. Furthermore, PINK1-deficient mice were protected against mitochondrial dysfunction, defects in mucociliary clearance, and airspace enlargement after CS exposure (207). Other evidence points to increased mitochondrial iron uptake promoting CS-induced mitochondrial dysfunction, as mice deficient for iron-responsive element-binding protein 2 (IRP2) were also protected from mitochondrial and airway dysfunction after CS inhalation exposure. Supporting the translatability of this finding, IREB2, the gene encoding IRP2, has been identified as a COPD susceptibility gene in humans (206, 210). How these mitochondrial regulators affect the response to air pollutants remains to be determined.
Epithelial dysfunction and senescence. The lung epithelium is the primary contact for inhaled pathogens and toxins and, thus, plays an important role in barrier function, pathogen clearance, and innate immunity (211). Specialized epithelial populations line the respiratory tract that contribute not only to gas exchange, but also to mucus production and removal of pathogens by mucociliary clearance. Homeostasis of the lung epithelium is regulated by region-specific regenerative programs that restore homeostasis after injury (212). Dysregulation of the immune and regenerative functions of the lung epithelium can contribute to disease (213).
Impaired barrier function has been proposed as a mechanism by which environmental exposures promote allergic diseases, including asthma (214). Exposure to ambient particles disrupts barrier integrity in cultured airway epithelial cells through downregulation of tight junction protein expression (215–218). Increasing cellular antioxidant capacity reduces inflammatory gene expression and prevents barrier loss, suggesting a link between oxidative stress, inflammation, and barrier integrity (215–217). Similar reductions in tight junction function have been observed in vivo (219), and mice exposed to intranasal or intratracheal particles from ambient air or diesel exhaust exhibited significantly elevated allergic responses to subsequent allergen exposure, including eosinophil infiltration, mucus metaplasia, and sneezing (219–221).
Mucociliary clearance is another major defense mechanism affected by air pollution. Studies in rabbits and rats have shown mucous metaplasia and ciliary abnormalities after exposure to either CAPs or wood smoke (222, 223). This is consistent with in vitro studies on human airway epithelial cultures, which have shown that particles from ambient air or diesel exhaust increase expression of mucus secretion genes while ciliary genes and beat frequency are reduced (224, 225). Impaired mucociliary function can promote respiratory infections and is implicated in the pathogenesis of COPD (226, 227).
Senescence affects the ability of the lung to regenerate after injury and is a hallmark of lung fibrosis (228, 229). Exposure to ambient particles induces senescence in cultured lung epithelial cells and fibroblasts (230, 231). Moreover, growth of alveolar organoids was impaired after exposure to diesel exhaust particles (232). While effects of air pollution exposure on lung senescence have yet to be demonstrated in vivo, PM2.5 and black carbon exposure have been shown to be inversely correlated with telomere length in circulating blood cells (233–235). Thus, it is likely that air pollution exposure affects senescence and regenerative responses in the lung.
Inflammatory responses to air pollution may also impair lung regeneration and promote fibrosis. Epithelial injury in the lung leads to development of a keratin 5–expressing, migratory, “fluid” epithelial phenotype that promotes wound closure (236). Dysregulation and persistence of this fluid phenotype is found in PF and was shown to be promoted by IL-6 (237). Lung and circulating IL-6 levels increased in mice after exposure to CAPs, and IL-6 plasma levels correlated with PM exposure in humans (171, 173, 186, 238), potentially providing a link between exposure, epithelial remodeling, and lung fibrosis.
Altered immune response. Air pollution exposure also affects how the immune populations of the lung respond to inhaled pathogens. AMs respond to PM exposure with upregulation of inflammatory cytokines including IL-1β, IL-6, IL-8, TNF-α, and GM-CSF (171, 239). Elimination of AMs in mice prevented pulmonary and systemic increases in IL-6 and TNF-α after either inhalation of CAPs or intranasal instillation of ambient particles (238, 240). However, long-term exposure to particles by intranasal instillation has been shown to decrease the ability of AMs to secrete IL-6 and IL-1β, resulting in increased death in mice subsequently exposed to influenza (241). In vitro cytokine induction (including IL-6, IFN-β, IL-1β, and TNF-α) after lipopolysaccharide or virus exposure was also blunted by previous exposure to particles from ambient air or diesel exhaust (242–244). Moreover, the ability of macrophages to conduct phagocytosis was impaired by CAP particle exposure (245). These changes suggest that PM causes a state of immune insensitivity, which may predispose to pulmonary infections.
PM exposure also affects lymphocyte populations in the lung. Dendritic cells exposed to ambient particles promote naive CD4+ T cell proliferation but with a reduced proportion of Th1 effectors (246). A similar reduction in Th1 cells was observed after in vivo inhalation exposure to particles, which correlated with more severe influenza infection (247). Other in vivo coexposure studies using PM and allergens have shown that prior PM instillation promotes development of a mixed Th2/Th17 phenotype that may perpetuate asthmatic responses (248, 249).
Epigenetic changes. Air pollution exposure may affect multiple epigenetic modifications, including alterations in DNA methylation and histone modifications. These changes may result in transient, and potentially permanent, changes in gene expression affecting lung function and causing long-term effects on respiratory health.
Changes in histone modifications due to PM exposure have been demonstrated in vitro (250), in animal models (251), and human studies (252, 253). Relatively less is known about how these changes affect cellular function after exposure to pollution; however, decreased IL-6 secretion from macrophages after long-term in vitro exposure to particles was shown to be associated with altered histone methylation events in the IL-6 promoter after exposure (241).
Exposure to air pollutants has generally been shown to coincide with global hypomethylation of DNA in various tissues, particularly in DNA repetitive elements. Exposure of rats to traffic-related pollution resulted in hypomethylation of blood and lung tissue LINE-1 elements (251). Hypomethylation of circulating leukocyte LINE-1 elements was shown in humans to correlate with black carbon exposure (254, 255). Total blood cell deoxycytidine methylation and total CpG site methylation was also negatively correlated with residential PM2.5 levels (256) and with diesel exhaust exposure in a controlled setting (257). At the gene level, differential methylation of gene elements has been shown to increase or decrease depending on gene and exposure (258, 259). One repeated finding is that exposure to ambient air pollution, diesel exhaust, or secondhand smoke is associated with hypermethylation of the FOXP3 gene, which leads to suppression of regulatory T cell function and increased asthma severity (260–262).
Carrier effects. As discussed above, air pollution can disrupt the airway epithelial barrier, impair mucociliary clearance of pathogens, and impair immune responses, all of which cause greater susceptibility to viral infection; however, air pollution particles themselves are also carriers of virus that can influence viral infectivity. Infective influenza virus can be transmitted between animals on nonrespiratory particles (263). Moreover, a study measuring ambient influenza virus in Taiwan found that ambient virus was significantly higher on days in which air particulates were elevated due to Asian dust storms (264). In a recent study, airborne ambient particles were shown to bind to influenza virus and promote cellular viral uptake in a receptor-independent manner. Furthermore, after nasal instillation, particle-associated virus was taken up deeper into the lung than virus alone, causing greater inflammation and sickness (265).
-
Conclusions and future directions
The current evidence shows that air pollution exposure is a major modifiable risk factor for the prevention and management of respiratory disease. As there is no “safe” level of air pollution exposure, efforts to reduce air pollution production will need to be combined with mitigation strategies. Future directions that need to be taken to improve our understanding and to reduce the impact of air pollution on human health are summarized in Table 2. A greater mechanistic understanding of the toxic effects of air pollutants on the lung and other tissues will be required to develop strategies to combat the harmful effects of air pollution exposure. Recent advancements in single-cell transcriptomic and epigenomic techniques will likely play a major role in increasing the understanding of cellular and organismal response to inhaled pollutants. Furthermore, as the associations of air pollution exposure with pulmonary disease become increasingly clear, increased integration of exposure studies with disease modeling studies may help to elucidate the mechanisms by which exposure promotes pulmonary disease.
Advancements in the measurement of air pollutants will be crucial to aid epidemiologic studies by allowing for accurate quantitative assessment of pollutant exposure at greater resolution. While older studies relied on pollutant measurements from the nearest ground-based monitor, often categorizing exposure based on zip code, actual pollutant levels can vary greatly within these areas. Statistical modeling advances such as land use regression analysis and technological advances such as satellite monitoring have increased the resolution of epidemiologic studies; however, these advances still lack fine scale resolution, and data gaps exist due to cloudy days, for example (266). Continued development of low-cost monitor networks may help to increase study resolution and allow for integration of indoor exposures to data sets (267). Finally, studies capable of identifying specific pollutants in the complex mixture that are particularly toxic will also be required to mitigate the effects of air pollution exposure. Analysis of PM elemental composition by the ELAPSE study has shown that V content of particles is most consistently associated with mortality as well as lung cancer incidence (268, 269). Such advancements in epidemiologic analysis may inform future toxicological studies as well as lead to policy changes that limit specific pollutants. These future studies will benefit society as a whole, but they will have an outsized effect on vulnerable populations, including the aged and low-income populations, on whom the effects of reducing air pollution production have been most acutely demonstrated (270, 271).
-
Acknowledgments
RBH was supported by NIH grant R01HL151680. GMM was supported by NIH grants R01ES015024 and P30ES027792 and Department of Defense grants W81XWH2210387 and HT9425-24-1-0138. The authors would like to thank Vinayak Dravid and Northwestern University Atomic and Nanoscale Characterization Experimental (NUANCE) Center for their assistance with the electron microscopy images of particulate matter.
Address correspondence to: Gökhan M. Mutlu, The University of Chicago, Section of Pulmonary and Critical Care Medicine, 5841 S. Maryland Avenue, MC6026, Chicago, Illinois 60637, USA. Phone: 773.702.1002; Email: gmutlu@uchicago.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Hamanaka et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(17):e194312. https://doi.org/10.1172/JCI194312.
-
References
- Collaborators GBDF. Burden of disease scenarios for 204 countries and territories, 2022-2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2204–2256.
- Collaborators GBDRF. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2162–2203.
- Hamanaka RB, Mutlu GM. Particulate matter air pollution: effects on the cardiovascular system. Front Endocrinol (Lausanne). 2018;9:680.
- Newby DE, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83–93b.
- Brook RD, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378.
- Brook RD, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671.
- Bell ML, et al. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–995.
- Pope CA, 3rdEpidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environ Health Perspect. 2000;108 Suppl 4(suppl 4):713–723.
- Miller MR, et al. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8(4):577–602.
- Chen B, Kan H. Air pollution and population health: a global challenge. Environ Health Prev Med. 2008;13(2):94–101.
- Yun X, et al. Residential solid fuel emissions contribute significantly to air pollution and associated health impacts in China. Sci Adv. 2020;6(44):eaba7621.
- United States Environmental Protection Agency. NAAQS Table. https://www.epa.gov/criteria-air-pollutants/naaqs-table Updated July 31, 2025. Accessed August 6, 2025.
- Bell ML, et al. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environ Health Perspect. 2004;112(1):6–8.
- Stone R. Air pollution. Counting the cost of London’s killer smog. Science. 2002;298(5601):2106–2107.
- Samet JM, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med. 2000;343(24):1742–1749.
- Peng RD, et al. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161(6):585–594.
- Katsouyanni K, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12(5):521–531.
- Katsouyanni K, et al. Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air Pollution and Health: a European Approach. BMJ. 1997;314(7095):1658–1663.
- Samoli E, et al. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study. Environ Health Perspect. 2008;116(11):1480–1486.
- Bell ML, et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999-2005. Am J Epidemiol. 2008;168(11):1301–1310.
- Atkinson RW, et al. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69(7):660–665.
- Liu C, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381(8):705–715.
- Liu C, et al. Coarse particulate air pollution and daily mortality: a global study in 205 cities. Am J Respir Crit Care Med. 2022;206(8):999–1007.
- Pope CA, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120(11):941–948.
- Burnett RT, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122(4):397–403.
- Apte JS, et al. Addressing global mortality from ambient PM2.5. Environ Sci Technol. 2015;49(13):8057–8066.
- Di Q, et al. Air pollution and mortality in the medicare population. N Engl J Med. 2017;376(26):2513–2522.
- Weichenthal S, et al. How low can you go? Air pollution affects mortality at very low levels. Sci Adv. 2022;8(39):eabo3381.
- Stafoggia M, et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health. 2022;6(1):e9–e18.
- Chen J, et al. Long-term exposure to low-level PM2.5 and mortality: investigation of heterogeneity by harmonizing analyses in large cohort studies in Canada, United States, and Europe. Environ Health Perspect. 2023;131(12):127003. View this article via: CrossRef Google Scholar
- Crouse DL, et al. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the canadian census health and environment cohort (CanCHEC). Environ Health Perspect. 2015;123(11):1180–1186.
- Dockery DW, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759.
- Pope CA, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151(3 pt 1):669–674.
- Beelen R, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383(9919):785–795.
- Laden F, et al. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173(6):667–672.
- Pope CA, et al. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360(4):376–386.
- Dominici F, et al. Particulate air pollution and mortality in the United States: did the risks change from 1987 to 2000? Am J Epidemiol. 2007;166(8):880–888.
- Huang J, et al. Health impact of China’s Air Pollution Prevention and Control Action Plan: an analysis of national air quality monitoring and mortality data. Lancet Planet Health. 2018;2(7):313–323.
- Wang W, et al. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Environ Sci Technol. 2009;43(14):5314–5320.
- Huang W, et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012;186(11):1150–1159.
- Rich DQ, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307(19):2068–2078.
- Li Y, et al. Impact of air pollution control measures and weather conditions on asthma during the 2008 Summer Olympic Games in Beijing. Int J Biometeorol. 2011;55(4):547–554.
- Li Y, et al. Air quality and outpatient visits for asthma in adults during the 2008 Summer Olympic Games in Beijing. Sci Total Environ. 2010;408(5):1226–1227.
- Su C, et al. Short-term effects of fine particulate air pollution on cardiovascular hospital emergency room visits: a time-series study in Beijing, China. Int Arch Occup Environ Health. 2016;89(4):641–657.
- Su C, et al. Assessing responses of cardiovascular mortality to particulate matter air pollution for pre-, during- and post-2008 Olympics periods. Environ Res. 2015;142:112–122.
- He G, et al. The effect of air pollution on mortality in China: Evidence from the 2008 Beijing Olympic Games. J Environ Econ Manag. 2016;79:18–39.View this article via: CrossRef Google Scholar
- Breitner S, et al. The association between particulate air pollution and respiratory mortality in Beijing before, during, and after the 2008 Olympic and Paralympic Games. Front Environ Sci. 2021;9:624180.
- Venter ZS, et al. COVID-19 lockdowns cause global air pollution declines. Proc Natl Acad Sci U S A. 2020;117(32):18984–18990.
- Qi J, et al. Short- and medium-term impacts of strict anti-contagion policies on non-COVID-19 mortality in China. Nat Hum Behav. 2022;6(1):55–63.
- Riou J, et al. Direct and indirect effects of the COVID-19 pandemic on mortality in Switzerland. Nat Commun. 2023;14(1):90.
- Giani P, et al. Short-term and long-term health impacts of air pollution reductions from COVID-19 lockdowns in China and Europe: a modelling study. Lancet Planet Health. 2020;4(10):e474–e482.
- Chen K, et al. Air pollution reduction and mortality benefit during the COVID-19 outbreak in China. Lancet Planet Health. 2020;4(6):210–212.
- Chossiere GP, et al. Air pollution impacts of COVID-19-related containment measures. Sci Adv. 2021;7(21):eabe1178.
- Burke M, et al. The contribution of wildfire to PM2.5 trends in the USA. Nature. 2023;622(7984):761–766.
- Ma Y, et al. Long-term exposure to wildland fire smoke PM2.5 and mortality in the contiguous United States. Proc Natl Acad Sci U S A. 2024;121(40):e2403960121.
- Ye T, et al. Short-term exposure to wildfire-related PM2.5 increases mortality risks and burdens in Brazil. Nat Commun. 2022;13(1):7651.
- Dohrenwend PB, et al. The impact on emergency department visits for respiratory illness during the southern california wildfires. West J Emerg Med. 2013;14(2):79–84.
- Delfino RJ, et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup Environ Med. 2009;66(3):189–197.
- Aguilera R, et al. Wildfire smoke impacts respiratory health more than fine particles from other sources: observational evidence from Southern California. Nat Commun. 2021;12(1):1493.
- Connolly R, et al. Mortality attributable to PM2.5 from wildland fires in California from 2008 to 2018. Sci Adv. 2024;10(23):eadl1252.
- McArdle CE, et al. Asthma-associated emergency department visits during the Canadian Wildfire Smoke Episodes - United States, April- August 2023. MMWR Morb Mortal Wkly Rep. 2023;72(34):926–932.
- Chen K, et al. Canadian wildfire smoke and asthma syndrome emergency department visits in New York City. JAMA. 2023;330(14):1385–1387.
- Maldarelli ME, et al. Polluted air from Canadian wildfires and cardiopulmonary disease in the Eastern US. JAMA Netw Open. 2024;7(12):e2450759.
- Landrigan PJ, et al. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112(6):731–739.
- Prezant DJ, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347(11):806–815.
- Chen LC, Thurston G. World Trade Center cough. Lancet. 2002;360 Suppl:s37–s38.
- Aldrich TK, et al. Lung function trajectories in World Trade Center-Exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149(6):1419–1427.
- Aldrich TK, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362(14):1263–1272.
- Thomas PA, et al. Respiratory and other health effects reported in children exposed to the World Trade Center disaster of 11 September 2001. Environ Health Perspect. 2008;116(10):1383–1390.
- Weiden MD, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137(3):566–574.
- Li J, et al. Pulmonary fibrosis among World Trade Center responders: results from the WTC health registry cohort. Int J Environ Res Public Health. 2019;16(5):825.
- Aldrich TK, et al. Bronchial reactivity and lung function after World Trade Center exposure. Chest. 2016;150(6):1333–1340.
- Liu S, et al. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J. 2021;57(6):2003099.
- Liu S, et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ Int. 2021;146:106267.
- Hvidtfeldt UA, et al. Long-term low-level ambient air pollution exposure and risk of lung cancer - A pooled analysis of 7 European cohorts. Environ Int. 2021;146:106249.
- Makar M, et al. Estimating the causal effect of low levels of fine particulate matter on hospitalization. Epidemiology. 2017;28(5):627–634.
- Koenig JQ, et al. The effects of inhaled sulfuric acid on pulmonary function in adolescent asthmatics. Am Rev Respir Dis. 1983;128(2):221–225.View this article via: PubMed Google Scholar
- Utell MJ, et al. Airway reactivity to sulfate and sulfuric acid aerosols in normal and asthmatic subjects. J Air Pollut Control Assoc. 1984;34(9):931–935.
- Koenig JQ, et al. Acute effects of inhaled SO2 plus NaCl droplet aerosol on pulmonary function in asthmatic adolescents. Environ Res. 1980;22(1):145–153.
- Forsberg B, et al. Daily air pollution levels and acute asthma in southern Sweden. Eur Respir J. 1998;12(4):900–905.
- Segala C, et al. Short-term effect of winter air pollution on respiratory health of asthmatic children in Paris. Eur Respir J. 1998;11(3):677–685.
- Ostro B, et al. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12(2):200–208.
- Gent JF, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290(14):1859–1867.
- Norris G, et al. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect. 1999;107(6):489–493.
- Strickland MJ, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182(3):307–316.
- Mar TF, et al. Associations between asthma emergency visits and particulate matter sources, including diesel emissions from stationary generators in Tacoma, Washington. Inhal Toxicol. 2010;22(6):445–448.
- Tian Y, et al. Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ Pollut. 2017;230:227–233.
- Sheffield PE, et al. Ambient ozone exposure and children’s acute asthma in New York City: a case-crossover analysis. Environ Health. 2015;14:25.
- Chen K, et al. The effects of air pollution on asthma hospital admissions in Adelaide, South Australia, 2003-2013: time-series and case-crossover analyses. Clin Exp Allergy. 2016;46(11):1416–1430.
- Wei Y, et al. Air pollutants and asthma hospitalization in the medicaid population. Am J Respir Crit Care Med. 2022;205(9):1075–1083.
- Pope CA, 3rdRespiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79(5):623–628.
- Pope CA, 3rdRespiratory hospital admissions associated with PM10 pollution in Utah, Salt Lake, and Cache Valleys. Arch Environ Health. 1991;46(2):90–97.
- Sbihi H, et al. Asthma trajectories in a population-based birth cohort. Impacts of air pollution and greenness. Am J Respir Crit Care Med. 2017;195(5):607–613.
- Rice MB, et al. Lifetime air pollution exposure and asthma in a pediatric birth cohort. J Allergy Clin Immunol. 2018;141(5):1932–1934.
- Jung CR, et al. Fine particulate matter exposure during pregnancy and infancy and incident asthma. J Allergy Clin Immunol. 2019;143(6):2254–2262.
- Gehring U, et al. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J. 2020;56(1):2000147.
- Peters JM, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159(3):768–775.
- Gauderman WJ, et al. Association between air pollution and lung function growth in southern California children: results from a second cohort. Am J Respir Crit Care Med. 2002;166(1):76–84.
- Gauderman WJ, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067.
- Gauderman WJ, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369(9561):571–577.
- Islam T, et al. Relationship between air pollution, lung function and asthma in adolescents. Thorax. 2007;62(11):957–963.
- Avol EL, et al. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164(11):2067–2072.
- Gauderman WJ, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–913.
- Garcia E, et al. Association of changes in air quality with incident asthma in children in California, 1993-2014. JAMA. 2019;321(19):1906–1915.
- Pope CA, et al. Acute effects of PM10 pollution on pulmonary function of smokers with mild to moderate chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;147(6 pt 1):1336–1340.
- Harre ES, et al. Respiratory effects of air pollution in chronic obstructive pulmonary disease: a three month prospective study. Thorax. 1997;52(12):1040–1044.
- Silkoff PE, et al. Winter air pollution and disease parameters in advanced chronic obstructive pulmonary disease panels residing in Denver, Colorado. J Allergy Clin Immunol. 2005;115(2):337–344.
- Evangelopoulos D, et al. Personal exposure to air pollution and respiratory health of COPD patients in London. Eur Respir J. 2021;58(1):2003432.
- Schwartz J. Air pollution and hospital admissions for the elderly in Detroit, Michigan. Am J Respir Crit Care Med. 1994;150(3):648–655.
- Schwartz J. Air pollution and hospital admissions for the elderly in Birmingham, Alabama. Am J Epidemiol. 1994;139(6):589–598.
- Anderson HR, et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997;10(5):1064–1071.
- Arbex MA, et al. Urban air pollution and chronic obstructive pulmonary disease-related emergency department visits. J Epidemiol Community Health. 2009;63(10):777–783.
- Gan WQ, et al. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187(7):721–727.
- Hoffmann C, et al. Asthma and COPD exacerbation in relation to outdoor air pollution in the metropolitan area of Berlin, Germany. Respir Res. 2022;23(1):64.
- Faustini A, et al. Short-term effects of air pollution in a cohort of patients with chronic obstructive pulmonary disease. Epidemiology. 2012;23(6):861–879.
- Doiron D, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54(1):1802140.
- Shin S, et al. Air pollution as a risk factor for incident chronic obstructive pulmonary disease and asthma. A 15-year population-based Cohort Study. Am J Respir Crit Care Med. 2021;203(9):1138–1148.
- Guo C, et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2(3):114–125.
- Haythorn SR, Meller HB. Necropsy evidences on the relation of smoky atmosphere to pneumonia. Am J Public Health Nations Health. 1938;28(4):479–486.
- Dominici F, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134.
- MacIntyre EA, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect. 2014;122(1):107–113.
- Landguth EL, et al. The delayed effect of wildfire season particulate matter on subsequent influenza season in a mountain west region of the USA. Environ Int. 2020;139:105668.
- Yu LJ, et al. Short-term exposure to ambient air pollution and influenza: a multicity study in China. Environ Health Perspect. 2023;131(12):127010. View this article via: CrossRef Google Scholar
- Yang J, et al. Influence of air pollution on influenza-like illness in China: a nationwide time-series analysis. EBioMedicine. 2023;87:104421.
- Horne BD, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med. 2018;198(6):759–766.
- Karr CJ, et al. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res. 2009;109(3):321–327.
- Travaglio M, et al. Links between air pollution and COVID-19 in England. Environ Pollut. 2021;268(pt a):115859.
- Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut. 2020;264:114732.
- Chen N, et al. Impact of air pollutants on pediatric admissions for Mycoplasma pneumonia: a cross-sectional study in Shanghai, China. BMC Public Health. 2020;20(1):447.
- Lai TC, et al. Ambient air pollution and risk of tuberculosis: a cohort study. Occup Environ Med. 2016;73(1):56–61.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349.
- Matthay MA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18.
- Reilly JP, et al. Low to moderate air pollutant exposure and acute respiratory distress syndrome after severe trauma. Am J Respir Crit Care Med. 2019;199(1):62–70.
- Gutman L, et al. Long-term exposure to ambient air pollution is associated with an increased incidence and mortality of acute respiratory distress syndrome in a large French region. Environ Res. 2022;212(pt d):113383.
- Chen Z, et al. Ambient air pollutant exposures and COVID-19 severity and mortality in a cohort of patients with COVID-19 in Southern California. Am J Respir Crit Care Med. 2022;206(4):440–448.
- Bozack A, et al. Long-term air pollution exposure and COVID-19 mortality: a patient-level analysis from New York City. Am J Respir Crit Care Med. 2022;205(6):651–662.
- Kim H, Bell ML. Air pollution and COVID-19 mortality in New York City. Am J Respir Crit Care Med. 2021;204(1):97–99.
- Stafoggia M, et al. Long-term exposure to ambient air pollution and mortality among four million COVID-19 cases in Italy: The EpiCovAir Study. Environ Health Perspect. 2023;131(5):57004. View this article via: CrossRef Google Scholar
- Zhou X, et al. Excess of COVID-19 cases and deaths due to fine particulate matter exposure during the 2020 wildfires in the United States. Sci Adv. 2021;7(33):eabi8789.
- Johannson KA, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43(4):1124–1131.
- Johannson KA, et al. Air pollution exposure is associated with lower lung function, but not changes in lung function, in patients with idiopathic pulmonary fibrosis. Chest. 2018;154(1):119–125.
- Winterbottom CJ, et al. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. 2018;153(5):1221–1228.
- Sese L, et al. Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax. 2018;73(2):145–150.
- Goobie GC, et al. Ambient ultrafine particulate matter and clinical outcomes in fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2024;209(9):1082–1090.View this article via: CrossRef Google Scholar
- Dales R, et al. The association between air pollution and hospitalization of patients with idiopathic pulmonary fibrosis in Chile: a daily time series analysis. Chest. 2020;158(2):630–636.
- Cui F, et al. Air pollutants, genetic susceptibility and risk of incident idiopathic pulmonary fibrosis. Eur Respir J. 2023;61(2):2200777.
- Sack C, et al. Air pollution and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA) air-lung study. Eur Respir J. 2017;50(6):1700559.
- Rice MB, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019;74(11):1063–1069.
- LoPiccolo J, et al. Lung cancer in patients who have never smoked - an emerging disease. Nat Rev Clin Oncol. 2024;21(2):121–146.
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2(4682):739–748.
- Raaschou-Nielsen O, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14(9):813–822.
- Liu CS, et al. Long-term association of air pollution and incidence of lung cancer among older Americans: A national study in the Medicare cohort. Environ Int. 2023;181:108266.
- Pope CA, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141.
- Turner MC, et al. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med. 2011;184(12):1374–1381.
- Eckel SP, et al. Air pollution affects lung cancer survival. Thorax. 2016;71(10):891–898.
- Liu C, et al. The effect of ambient PM2.5 exposure on survival of lung cancer patients after lobectomy. Environ Health. 2023;22(1):23.
- Hallett AM, et al. Ambient air pollution and adverse waitlist events among lung transplant candidates. Transplantation. 2022;106(5):1071–1077.
- Ruttens D, et al. An association of particulate air pollution and traffic exposure with mortality after lung transplantation in Europe. Eur Respir J. 2017;49(1):1600484.
- Bhinder S, et al. Air pollution and the development of posttransplant chronic lung allograft dysfunction. Am J Transplant. 2014;14(12):2749–2757.
- Nawrot TS, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66(9):748–754.
- Benmerad M, et al. Chronic effects of air pollution on lung function after lung transplantation in the Systems prediction of Chronic Lung Allograft Dysfunction (SysCLAD) study. Eur Respir J. 2017;49(1):1600206.
- Choi D, et al. Pollution exposure in the first 3 months post transplant is associated with lower baseline FEV1 and higher CLAD risk. J Heart Lung Transplant. 2024;43(12):1987–1997.
- Amubieya O, et al. Ambient air pollution exposure and outcomes in patients receiving lung transplant. JAMA Netw Open. 2024;7(10):e2437148.
- Ghio AJ, Huang YC. Exposure to concentrated ambient particles (CAPs): a review. Inhal Toxicol. 2004;16(1):53–59.
- May WE, et al. Standard reference materials for chemical and biological studies of complex environmental samples. Mutat Res. 1992;276(1-2):11–22.
- Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674(1-2):45–54.
- Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612–616.
- Moller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect. 2010;118(8):1126–1136.
- Chuang KJ, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176(4):370–376.
- Ghio AJ, et al. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162(3 pt 1):981–988.
- van Eeden SF, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164(5):826–830.
- Riediker M, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940.
- Tsai DH, et al. Effects of particulate matter on inflammatory markers in the general adult population. Part Fibre Toxicol. 2012;9:24.
- Mutlu GM, et al. Airborne particulate matter inhibits alveolar fluid reabsorption in mice via oxidant generation. Am J Respir Cell Mol Biol. 2006;34(6):670–676.
- Soberanes S, et al. p53 mediates particulate matter-induced alveolar epithelial cell mitochondria-regulated apoptosis. Am J Respir Crit Care Med. 2006;174(11):1229–1238.
- Manzo ND, et al. Nitric oxide and superoxide mediate diesel particle effects in cytokine-treated mice and murine lung epithelial cells--implications for susceptibility to traffic-related air pollution. Part Fibre Toxicol. 2012;9:43.
- Hong Z, et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016;239(2):117–125.
- Wang J, et al. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J Thorac Dis. 2017;9(11):4398–4412.
- Li N, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111(4):455–460.
- Zhao Q, et al. Direct effects of airborne PM2.5 exposure on macrophage polarizations. Biochim Biophys Acta. 2016;1860(12):2835–2843.
- Li R, et al. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free Radic Biol Med. 2009;46(6):775–782.
- Montiel-Davalos A, et al. Oxidative stress and apoptosis are induced in human endothelial cells exposed to urban particulate matter. Toxicol In Vitro. 2010;24(1):135–141.
- London NR Jr. , et al. Air pollutant-mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J Allergy Clin Immunol. 2016;138(6):1736–1738.
- Chan JK, et al. Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs. Part Fibre Toxicol. 2013;10:34.
- Imrich A, et al. Alveolar macrophage cytokine response to air pollution particles: oxidant mechanisms. Toxicol Appl Pharmacol. 2007;218(3):256–264.
- Chiarella SE, et al. β2-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J Clin Invest. 2014;124(7):2935–2946.
- Tripathi P, et al. Variation in doses and duration of particulate matter exposure in bronchial epithelial cells results in upregulation of different genes associated with airway disorders. Toxicol In Vitro. 2018;51:95–105.
- Xu C, et al. High molecular weight hyaluronan attenuates fine particulate matter-induced acute lung injury through inhibition of ROS-ASK1-p38/JNK-mediated epithelial apoptosis. Environ Toxicol Pharmacol. 2018;59:190–198.
- Ge C, et al. Nrf2 deficiency aggravates PM2.5-induced cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation via RIPK3-regulated mitochondrial disorder. Aging (Albany NY). 2020;12(6):4836–4865.
- Vincenzo SD, et al. Oxidative stress, environmental pollution, and lifestyle as determinants of asthma in children. Biology (Basel). 2023;12(1):133. View this article via: PubMed Google Scholar
- Miklos Z, Horvath I. The role of oxidative stress and antioxidants in cardiovascular comorbidities in COPD. Antioxidants (Basel). 2023;12(6):1196.
- Barnes PJ, et al. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(1):6–14.
- Lambrecht BN, et al. The cytokines of asthma. Immunity. 2019;50(4):975–991.
- Zhou T, et al. Association between the oxidative stress gene polymorphism and chronic obstructive pulmonary disease risk: a meta-analysis. BMC Pulm Med. 2023;23(1):384.
- Salam MT, et al. Microsomal epoxide hydrolase, glutathione S-transferase P1, traffic and childhood asthma. Thorax. 2007;62(12):1050–1057.
- Hill W, et al. Lung adenocarcinoma promotion by air pollutants. Nature. 2023;616(7955):159–167.
- Huang Y, et al. Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK Biobank. Am J Respir Crit Care Med. 2021;204(7):817–825.
- Leclercq B, et al. Air pollution-derived PM2.5 impairs mitochondrial function in healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells. Environ Pollut. 2018;243(pt b):1434–1449.
- Li R, et al. Effect of ambient PM(2.5) on lung mitochondrial damage and fusion/fission gene expression in rats. Chem Res Toxicol. 2015;28(3):408–418.
- Jin X, et al. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J Toxicol Sci. 2018;43(2):101–111.
- Caceres L, et al. NADPH oxidase and mitochondria are relevant sources of superoxide anion in the oxinflammatory response of macrophages exposed to airborne particulate matter. Ecotoxicol Environ Saf. 2020;205:111186.
- Guo Z, et al. PM(2.5)-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int J Environ Res Public Health. 2017;14(2):134.
- Soberanes S, et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem. 2009;284(4):2176–2186.
- Soberanes S, et al. Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis. Cell Metab. 2019;29(2):335–347.
- van der Toorn M, et al. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1211–L1218.
- Cloonan SM, et al. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat Med. 2016;22(2):163–174.
- Mizumura K, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124(9):3987–4003.
- Hoffmann RF, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;14(1):97.
- Hara H, et al. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol. 2013;305(10):737–746.
- DeMeo DL, et al. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. Am J Hum Genet. 2009;85(4):493–502.
- Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol. 2021;21(6):347–362.
- Planer JD, Morrisey EE. After the storm: regeneration, repair, and reestablishment of homeostasis between the alveolar epithelium and innate immune system following viral lung injury. Annu Rev Pathol. 2023;18:337–359.
- Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021;14(5):978–990.
- Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–751.
- Lee DC, et al. Urban particulate matter regulates tight junction proteins by inducing oxidative stress via the Akt signal pathway in human nasal epithelial cells. Toxicol Lett. 2020;333:33–41.
- Caraballo JC, et al. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology. 2011;16(2):340–349.
- Zhao R, et al. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J Appl Toxicol. 2018;38(5):678–687.
- Xian M, et al. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol Res. 2020;12(1):56–71.
- Fukuoka A, et al. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016;46(1):142–152.
- Wang T, et al. Murine lung responses to ambient particulate matter: genomic analysis and influence on airway hyperresponsiveness. Environ Health Perspect. 2008;116(11):1500–1508.
- Zhao C, et al. Respiratory exposure to PM2.5 soluble extract disrupts mucosal barrier function and promotes the development of experimental asthma. Sci Total Environ. 2020;730:139145.
- Zhao R, et al. Effects of PM2.5 on mucus secretion and tissue remodeling in a rabbit model of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(11):1349–1355.
- He F, et al. Exposure to ambient particulate matter induced COPD in a rat model and a description of the underlying mechanism. Sci Rep. 2017;7:45666.
- Bayram H, et al. Comparison of ciliary activity and inflammatory mediator release from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients and the effect of diesel exhaust particles in vitro. J Allergy Clin Immunol. 1998;102(5):771–782.
- Montgomery MT, et al. Genome-wide analysis reveals mucociliary remodeling of the nasal airway epithelium induced by urban PM2.5. Am J Respir Cell Mol Biol. 2020;63(2):172–184.
- Bhowmik A, et al. Improving mucociliary clearance in chronic obstructive pulmonary disease. Respir Med. 2009;103(4):496–502.
- Fu Y, et al. Ciliostasis of airway epithelial cells facilitates influenza A virus infection. Vet Res. 2018;49(1):65.
- Liu RM, Liu G. Cell senescence and fibrotic lung diseases. Exp Gerontol. 2020;132:110836.
- Hernandez-Gonzalez F, et al. Cellular senescence in lung fibrosis. Int J Mol Sci. 2021;22(13):7012.
- Jin S, et al. Antioxidants prevent particulate matter-induced senescence of lung fibroblasts. Heliyon. 2023;9(3):e14179.
- Chang-Chien J, et al. Particulate matter causes telomere shortening and increase in cellular senescence markers in human lung epithelial cells. Ecotoxicol Environ Saf. 2021;222:112484.
- Wu X, et al. Diesel exhaust particles distort lung epithelial progenitors and their fibroblast niche. Environ Pollut. 2022;305:119292.
- Dioni L, et al. Effects of short-term exposure to inhalable particulate matter on telomere length, telomerase expression, and telomerase methylation in steel workers. Environ Health Perspect. 2011;119(5):622–627.
- McCracken J, et al. Annual ambient black carbon associated with shorter telomeres in elderly men: Veterans Affairs Normative Aging Study. Environ Health Perspect. 2010;118(11):1564–1570.
- Pieters N, et al. Biomolecular markers within the core axis of aging and particulate air pollution exposure in the elderly: a cross-sectional study. Environ Health Perspect. 2016;124(7):943–950.
- Park JA, et al. Collective migration and cell jamming in asthma, cancer and development. J Cell Sci. 2016;129(18):3375–3383.
- Stancil IT, et al. Interleukin-6-dependent epithelial fluidization initiates fibrotic lung remodeling. Sci Transl Med. 2022;14(654):eabo5254.
- Mutlu GM, et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117(10):2952–2961.
- Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171(1):20–26.
- Marchini T, et al. Acute exposure to air pollution particulate matter aggravates experimental myocardial infarction in mice by potentiating cytokine secretion from lung macrophages. Basic Res Cardiol. 2016;111(4):44.
- Ma JH, et al. Long-term exposure to PM2.5 lowers influenza virus resistance via down-regulating pulmonary macrophage Kdm6a and mediates histones modification in IL-6 and IFN-β promoter regions. Biochem Biophys Res Commun. 2017;493(2):1122–1128.
- Castranova V, et al. Effect of exposure to diesel exhaust particles on the susceptibility of the lung to infection. Environ Health Perspect. 2001;109 Suppl 4(suppl 4):609–612.
- Becker S, Soukup JM. Exposure to urban air particulates alters the macrophage-mediated inflammatory response to respiratory viral infection. J Toxicol Environ Health A. 1999;57(7):445–457.
- Tao RJ, et al. PM2.5 compromises antiviral immunity in influenza infection by inhibiting activation of NLRP3 inflammasome and expression of interferon-β. Mol Immunol. 2020;125:178–186.
- Li Y, et al. Fine particulate matter inhibits phagocytosis of macrophages by disturbing autophagy. FASEB J. 2020;34(12):16716–16735.
- Matthews NC, et al. Urban particulate matter suppresses priming of T helper type 1 cells by granulocyte/macrophage colony-stimulating factor-activated human dendritic cells. Am J Respir Cell Mol Biol. 2014;50(2):281–291.
- Lee GI, et al. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part Fibre Toxicol. 2014;11:57.
- Brandt EB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132(5):1194–1204.
- Wang P, et al. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45(5):977–983.
- Gilmour PS, et al. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol. 2003;284(3):L533–L540.
- Ding R, et al. Dose- and time- effect responses of DNA methylation and histone H3K9 acetylation changes induced by traffic-related air pollution. Sci Rep. 2017;7:43737.
- Liu C, et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ Health. 2015;14:65.
- Zheng Y, et al. Traffic-derived particulate matter exposure and histone H3 modification: A repeated measures study. Environ Res. 2017;153:112–119.
- Baccarelli A, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–578.
- Madrigano J, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect. 2011;119(7):977–982.
- De Prins S, et al. Influence of ambient air pollution on global DNA methylation in healthy adults: a seasonal follow-up. Environ Int. 2013;59:418–424.
- Jiang R, et al. Short-term diesel exhaust inhalation in a controlled human crossover study is associated with changes in DNA methylation of circulating mononuclear cells in asthmatics. Part Fibre Toxicol. 2014;11:71.
- Wang M, et al. Genome-wide DNA methylation analysis reveals significant impact of long-term ambient air pollution exposure on biological functions related to mitochondria and immune response. Environ Pollut. 2020;264:114707.
- Xu R, et al. Wildfire-related PM(2.5) and DNA methylation: An Australian twin and family study. Environ Int. 2023;171:107704. View this article via: CrossRef Google Scholar
- Nadeau K, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–852.
- Brunst KJ, et al. Forkhead box protein 3 (FOXP3) hypermethylation is associated with diesel exhaust exposure and risk for childhood asthma. J Allergy Clin Immunol. 2013;131(2):592–594.
- Kohli A, et al. Secondhand smoke in combination with ambient air pollution exposure is associated with increasedx CpG methylation and decreased expression of IFN-γ in T effector cells and Foxp3 in T regulatory cells in children. Clin Epigenetics. 2012;4(1):17.
- Asadi S, et al. Influenza A virus is transmissible via aerosolized fomites. Nat Commun. 2020;11(1):4062.
- Chen PS, et al. Ambient influenza and avian influenza virus during dust storm days and background days. Environ Health Perspect. 2010;118(9):1211–1216.
- Dong Z, et al. Airborne fine particles drive H1N1 viruses deep into the lower respiratory tract and distant organs. Sci Adv. 2023;9(23):eadf2165.
- Ma X, et al. A comprehensive review of the development of land use regression approaches for modeling spatiotemporal variations of ambient air pollution: A perspective from 2011 to 2023. Environ Int. 2024;183:108430.
- Lunderberg DM, et al. Assessing residential PM2.5 concentrations and infiltration factors with high spatiotemporal resolution using crowdsourced sensors. Proc Natl Acad Sci U S A. 2023;120(50):e2308832120.
- Chen J, et al. Long-term exposure to fine particle elemental components and natural and cause-specific mortality-a pooled analysis of eight european cohorts within the ELAPSE Project. Environ Health Perspect. 2021;129(4):47009. View this article via: CrossRef Google Scholar
- Hvidtfeldt UA, et al. Long-term exposure to fine particle elemental components and lung cancer incidence in the ELAPSE pooled cohort. Environ Res. 2021;193:110568.
- Yazdi MD, et al. Long-term effect of exposure to lower concentrations of air pollution on mortality among US Medicare participants and vulnerable subgroups: a doubly-robust approach. Lancet Planet Health. 2021;5(10):e689–e697.
- Josey KP, et al. Air pollution and mortality at the intersection of race and social class. N Engl J Med. 2023;388(15):1396–1404.
- Pryor JT, et al. The physiological effects of air pollution: particulate matter, physiology and disease. Front Public Health. 2022;10:882569.
-
Version history
- Version 1 (September 2, 2025): Electronic publication



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)




