Advertisement
Commentary Free access | 10.1172/JCI17622
The intricate interplay among body weight, stress, and the immune response to friend or foe
Lawrence Steinman,1 Paul Conlon,2 Rich Maki,2 and Alan Foster2
1Interdepartmental Program in Immunology and Department of Neurological Sciences, Stanford University, Stanford, California, USA2 Neurocrine Biosciences, San Diego, California, USA
Address correspondence to: Lawrence Steinman, Interdepartmental Program in Immunology and the Department of Neurological Sciences, Stanford University, B002 Beckman Center for Molecular Medicine, Stanford, California 94205, USA. Phone: (650) 725-6401; Fax: (650) 725-0627; E-mail: steinman@stanford.edu.
Find articles by Steinman, L. in: PubMed | Google Scholar
1Interdepartmental Program in Immunology and Department of Neurological Sciences, Stanford University, Stanford, California, USA2 Neurocrine Biosciences, San Diego, California, USA
Address correspondence to: Lawrence Steinman, Interdepartmental Program in Immunology and the Department of Neurological Sciences, Stanford University, B002 Beckman Center for Molecular Medicine, Stanford, California 94205, USA. Phone: (650) 725-6401; Fax: (650) 725-0627; E-mail: steinman@stanford.edu.
Find articles by Conlon, P. in: PubMed | Google Scholar
1Interdepartmental Program in Immunology and Department of Neurological Sciences, Stanford University, Stanford, California, USA2 Neurocrine Biosciences, San Diego, California, USA
Address correspondence to: Lawrence Steinman, Interdepartmental Program in Immunology and the Department of Neurological Sciences, Stanford University, B002 Beckman Center for Molecular Medicine, Stanford, California 94205, USA. Phone: (650) 725-6401; Fax: (650) 725-0627; E-mail: steinman@stanford.edu.
Find articles by Maki, R. in: PubMed | Google Scholar
1Interdepartmental Program in Immunology and Department of Neurological Sciences, Stanford University, Stanford, California, USA2 Neurocrine Biosciences, San Diego, California, USA
Address correspondence to: Lawrence Steinman, Interdepartmental Program in Immunology and the Department of Neurological Sciences, Stanford University, B002 Beckman Center for Molecular Medicine, Stanford, California 94205, USA. Phone: (650) 725-6401; Fax: (650) 725-0627; E-mail: steinman@stanford.edu.
Find articles by Foster, A. in: PubMed | Google Scholar
Published January 15, 2003 - More info
J Clin Invest. 2003;111(2):183–185. https://doi.org/10.1172/JCI17622.
© 2003 The American Society for Clinical Investigation
We all know that when we suffer from fever we feel sleepy and do not feel like eating. Our mothers and grandmothers provided sound wisdom during our bouts of illness with fever. They recommended rest and tried to make us eat, advocating folk remedies like chicken soup or various teas. The physiologic roles of mediators of fever such as IL-1, TNF-α, IL-6, and prostaglandins account for some of the physiological changes in the sleep/wake cycle and in appetite (1, 2). Prostaglandins are involved in the induction of sleep, while TNF-α triggers loss of appetite and pyrexia. However, we are now learning that the interconnections between molecules that modulate brain function and those that mediate the immune response are more intertwined than we had previously imagined.
-
Neuroendocrine modulation of the immune response
Leptin, a molecule that is critical in the regulation of energy balance and body weight, is a strong regulator of Th1 autoimmunity (3, 4). This is one of many examples of redundancy and overlapping roles of molecules within neuroendocrine systems and the immune system. For example, it has been shown that corticotropin-releasing factor (CRF), a master regulator of hypothalamic and pituitary function, has an autonomous effect on the immune system. CRF can downregulate Th1 autoimmunity (2, 5). The neuroendocrine system can have potent effects on the immune system. Basic behaviors such as fasting have potent influences on the induction of Th1 autoimmunity. A short fast can circumvent an attack of autoimmune paralysis (4). Thus, while “feeding a cold” may have salutary effects in combating a viral infection, starving an autoimmune disease may protect against immune damage.
While “simple” acts such as fasting influence immunity, complicated states like pregnancy modulate autoimmune diseases as strongly as any known drug (2, 6). Gender itself has an impact on autoimmunity, females being far more susceptible than males to diseases like systemic lupus erythematous, rheumatoid arthritis, autoimmune thyroiditis, and MS (2). Interestingly, leptin is produced to a much greater extent by females than by males (3). This may in part account for the increased susceptibility of females to many organ-specific autoimmune diseases. However, CRF activity in the hypothalamic pituitary axis is generally higher in females. Given the inhibitory effect of CRF on autoimmune disease (5), an apparent paradox emerges, probably masking another layer of complexity, unexplained for now.
-
Leptin and CRF downregulate Th1 autoimmunity
In the current issue of the JCI, Matarese and colleagues describe the potent effects of leptin on Th1 autoimmunity, including the differential production of this molecule in females and the remarkable beneficial effect of a short fast on downregulating an incipient attack of autoimmunity (4). The authors demonstrate that leptin is produced at the site of autoimmune pathology. Neurons producing leptin may actually alter immune function, indicating direct neural control of an immune response (3, 4). One possible explanation for the link between leptin and control of the immune system is that during starvation, which results in low levels of leptin, the organism must conserve energy, and reduced leptin levels force the organism to reduce reproductive and thermogenic activities (7) while suppressing the immune system (8). It is worth noting that starvation also increases CRF expression, which also downregulates autoimmunity (5). CRF in turn upregulates cortisol, which also acts to dampen autoimmunity, though the effects of CRF on autoimmunity are still apparent even after adrenalectomy (5). Thus starvation, at least in the short term, has beneficial effects on autoimmunity, probably via the roles of leptin and CRF. The question also arises as to whether obesity and autoimmunity are comorbid conditions, given the fact that leptin levels are highly elevated in obese subjects.
Leptin is a member of the helical cytokine family, which includes IL-6, IL-11, IL-12, leukemia inhibitory factor, GCSF, ciliary neurotrophic factor, and oncostatin M (9, 10). The leptin receptor is a member of the class I cytokine receptor family, which includes gp-130, the common signal transducing component for the IL-6–related family of cytokines (9). Leptin mediates food intake, metabolism, and reproductive behavior. Leptin receptors are present on CD4+ T cells, where leptin mediates Th1 immunity. Mice with a deletion in the leptin gene (ob/ob mice) have a high level of thymocyte apoptosis with a marked reduction in the size and cellularity of the thymus (11). T cells in these mice produce minimal IFN-γ and a moderate amount of IL-4, resulting in an inability to generate a Th1 response while enhancing a Th2 phenotype (3, 4, 8).
-
Neuropeptides involved in body weight are found at sites of autoimmunity
There are indications that other neuroendocrine mediators such as the melanocortins (12, 13) and CRF (2, 12, 13) also have prominent roles in diseases like MS and rheumatoid arthritis, which are thought to be autoimmune in nature. Leptin is thought to produce its profound effects on appetite and body weight by altering the balance between the anorectic neuropeptides α-melano-cyte–stimulating hormone (α-MSH) and CRF and the orexigenic neuropeptides agouti-related peptide and neuropeptide Y. Shifts in the balance between these types of neuropetides also contribute to the cytokine-mediated loss of appetite and body weight, as well as malaise induced by lipopolysaccharide (14). One important aspect of this regulation is at the level of the pro-opiomelanocortin–expressing neurons in the arcuate nucleus of the hypothalamus, which are activated by leptin to release α-MSH (15). Apparently, this in-terplay of neuropeptides with re-gards to hypothalamic function may be reproduced at sites of autoimmune pathology (Figure 1). Large-scale analysis of gene transcripts in MS lesions revealed that leptin, melanocortin 4 receptor, and ACTH-receptor are elevated at the site of inflammation in the brain (12, 13). All three of these neuropeptides — leptin, melanocortin 4, and ACTH — have pleiotropic roles in neuroendocrine and immune physiology.
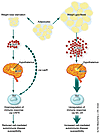 Figure 1
Figure 1Body fat and the autoimmune response. Decreases in body fat or starvation increase CRF levels and decrease leptin levels. Increased CRF downregulates Th1 immunity (5) as does decreased leptin (4). Other molecules of the α-MSH family stimulate Th1 T cells via their MC-4 receptor. CRFR, CRF receptor; LepR, leptin receptor.
The microsomal enzyme, stearoyl CoA desaturase–1 (SCD-1), is required for the biosynthesis of the monounsaturated fats, palmitoleate and oleate from saturated fatty acids (16). SCD-1 RNA levels are highly elevated in untreated ob/ob mouse liver, and indeed SCD-1 probably plays a decisive role in leptin’s metabolic effects. Interestingly, SCD-1 was downregulated in the MS brain as well (12), and both RNA levels and the activity of this enzyme are repressed by leptin (16). Ob/ob mice with mutations in SCD-1 are significantly less obese than ob/ob controls (16). The role of leptin in autoimmune brain disease and in the immune system in general may be mediated by downregulation of this enzyme involved in the biosynthesis of monosaturated fats. Once again we witness the remarkable choreography of molecules related to body weight and energy metabolism and the parallel roles of these same molecules in the finely tuned immune response.
Earlier work showed that CRF, the key regulator of the stress response in the hypothalamic-pituitary-adre-nal axis, or urocortin, a naturally occurring paralogue of CRF, acting directly on T cells in adrenalectomized mice, ameliorated experimental autoimmune encephalomye-litis. These effects were blocked by antagonists of CRF (5). Thus CRF, like leptin, is produced by the brain and may act directly on the immune system. The expression of CRF itself can be regulated by cytokines, adding yet another layer of complexity and a further target for intervention. Another neuropeptide, ACTH, a key mediator of the stress response produced in the pituitary gland, has been used for over 40 years to treat MS. ACTH receptor is expressed in the MS lesion itself (12).
These results imply that in autoimmunity, stress may be beneficial, and that short-term starvation may help reverse disease. In fighting microbial infections, perhaps stress is detrimental and eating is recommended, but in the case of autoimmunity, the opposite holds true: stress is actually good for you, and starvation is helpful. Grandmother’s advice may have to be refined depending on whether the immune system is attacking friend or foe.
-
Acknowledgments
The authors thank Wylie Vale for his insights and input.
-
Footnotes
See the related article beginning on page 241.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: corticotropin-releasing factor (CRF); α-melanocyte–stimulating hormone (α-MSH); stearoyl CoA desaturase–1 (SCD-1).
-
References
- Steinman, L. Connections between the immune system and the nervous system. Proc. Natl. Acad. Sci. USA. 1993. 90:7912-7914.
- Steinman, L. Autoimmune disease. Sci. Am. 1993. 269:106-114. View this article via: PubMed Google Scholar
- Matarese, G, et al. Requirement for leptin in induction and progression of experimental autoimmune encephalomyelitis. J. Immunol. 2001. 166:5909-5916. View this article via: PubMed Google Scholar
- Sanna, V, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J. Clin. Invest. 2003. 111:241-250. doi:10.1172/JCI200316721.
- Poliak, S, et al. Stress and autoimmunity: the neuropeptides corticotropin releasing factor and urocortin suppress encephalomyelitis via effects on both the hypothalamic-pituitary-adrenal axis and the immune system. J. Immunol. 1997. 158:5751-5756. View this article via: PubMed Google Scholar
- Langer-Gould, A, Garren, H, Slansky, A, Ruiz, PJ, Steinman, L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J. Immunol. 2002. 169:1084-1091. View this article via: PubMed Google Scholar
- Ahima, RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996. 382:250-252.
- Lord, GM, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998. 394:897-901.
- Baumann, H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA. 1996. 93:8374-8378.
- Zhang, F, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997. 387:206-209.
- Howard, J, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Invest. 1999. 104:1051-1059.
- Lock, C, et al. Gene microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002. 8:500-508.
- Steinman, L, Martin, R, Bernard, CCA, Conlon, P, Oksenberg, JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Ann. Rev. Neurosci. 2002. 25:491-505.
- Marks, DL, Ling, N, Cone, RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001. 61:1432-1438. View this article via: PubMed Google Scholar
- Cowley, MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001. 411:480-484.
- Cohen, P, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002. 297:240-243.
-
Version history
- Version 1 (January 15, 2003): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

