Felix Kreier, Eric Fliers, Peter J. Voshol, Corbert G. Van Eden, Louis M. Havekes, Andries Kalsbeek, Caroline L. Van Heijningen, Arja A. Sluiter, Thomas C. Mettenleiter, Johannes A. Romijn, Hans P. Sauerwein, Ruud M. Buijs
Felix Kreier, Eric Fliers, Peter J. Voshol, Corbert G. Van Eden, Louis M. Havekes, Andries Kalsbeek, Caroline L. Van Heijningen, Arja A. Sluiter, Thomas C. Mettenleiter, Johannes A. Romijn, Hans P. Sauerwein, Ruud M. Buijs
Abstract
Research Article
Authors
Felix Kreier, Eric Fliers, Peter J. Voshol, Corbert G. Van Eden, Louis M. Havekes, Andries Kalsbeek, Caroline L. Van Heijningen, Arja A. Sluiter, Thomas C. Mettenleiter, Johannes A. Romijn, Hans P. Sauerwein, Ruud M. Buijs
Figure 1
Options:
View larger image
(or click on image)
Download as PowerPoint
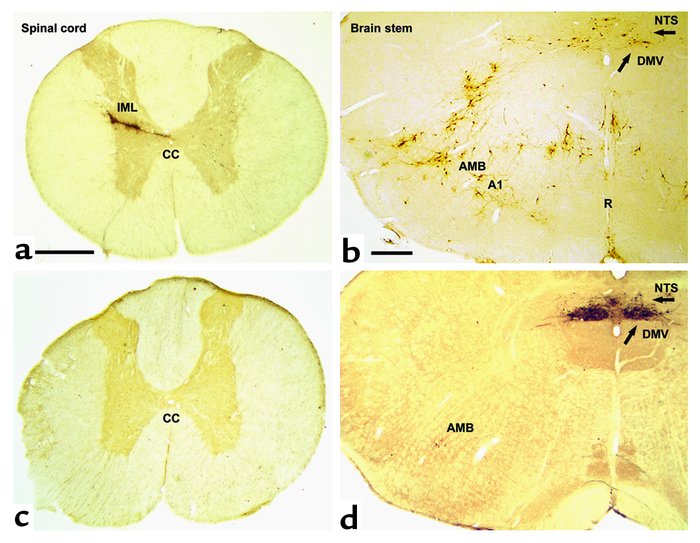
Transverse section of the spinal cord (at Th7) and the rat brain stem at the level of the obex shows spinal cord and brain stem neurons projecting into adipose tissue. Transneuronal retrograde tracing by PRV injection into retroperitoneal fat in rats before (a and b ) and after (c and d ) sympathetic denervation of adipose tissue. In a and b (PRV tracing from adipose tissue before denervation), since both sympathetic and parasympathetic fibers are intact, PRV is seen to spread via the vagus and the sympathetic nerves. Interestingly, the route via the IML is favored in intact animals such that second-order neurons in the brain stem are already evident when the first-order parasympathetic motor neurons appear in the DMV (arrow). In b , the A1 region, the raphe nucleus (R), and the nucleus of the solitary tract (NTS) project into the sympathetic motor neurons. In c (with d , showing PRV tracing after sympathetic denervation of the left retroperitoneal fat pad), there is no labeling of PRV in the IML. In the brain stem shown in d , neurons are clearly visible in the parasympathetic motor nuclei: DMV and caudal part of the AMB. CC, central canal. Bar in a and c = 0.5 mm. Bar in b and d = 0.4 mm.



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)