Abstract
Belying the spectacular success of solid organ transplantation and improvements in immunosuppressive therapy is the reality that long-term graft survival rates remain relatively unchanged, in large part due to chronic and insidious alloantibody-mediated graft injury. Half of heart transplant recipients develop chronic rejection within 10 years — a daunting statistic, particularly for young patients expecting to achieve longevity by enduring the rigors of a transplant. The current immunosuppressive pharmacopeia is relatively ineffective in preventing late alloantibody-associated chronic rejection. In this issue of the JCI, Kelishadi et al. report that preemptive deletion of B cells prior to heart transplantation in cynomolgus monkeys, in addition to conventional posttransplant immunosuppressive therapy with cyclosporine, markedly attenuated not only acute graft rejection but also alloantibody elaboration and chronic graft rejection. The success of this preemptive strike implies a central role for B cells in graft rejection, and this approach may help to delay or prevent chronic rejection after solid organ transplantation.
Authors
Stuart J. Knechtle, Jean Kwun, Neal Iwakoshi
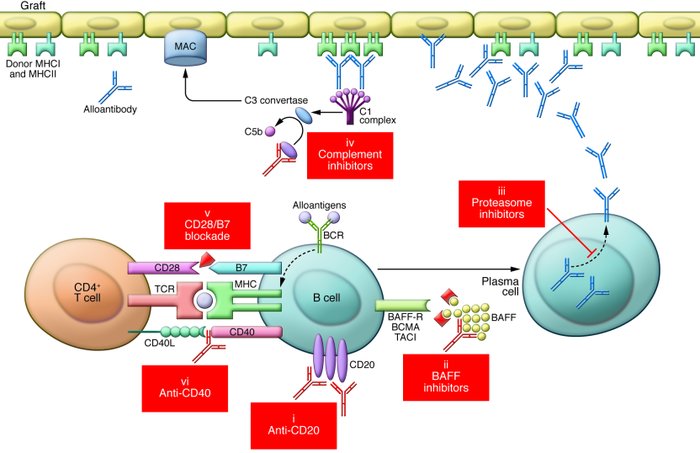
Figure 1
B cell– and antibody-related biologics in transplantation.



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)