Catherine Postic, Jean Girard
Citation Information: J Clin Invest. 2008;118(3):829-838. https://doi.org/10.1172/JCI34275.
Catherine Postic, Jean Girard
Published March 3, 2008
Citation Information: J Clin Invest. 2008;118(3):829-838. https://doi.org/10.1172/JCI34275.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with obesity, insulin resistance, and type 2 diabetes. NAFLD represents a large spectrum of diseases ranging from (i) fatty liver (hepatic steatosis); (ii) steatosis with inflammation and necrosis; and (iii) cirrhosis. Although the molecular mechanism leading to the development of hepatic steatosis in the pathogenesis of NAFLD is complex, recent animal models have shown that modulating important enzymes in fatty acid synthesis in liver may be key for the treatment of NAFLD. This review discusses recent advances in the field.
Authors
Catherine Postic, Jean Girard
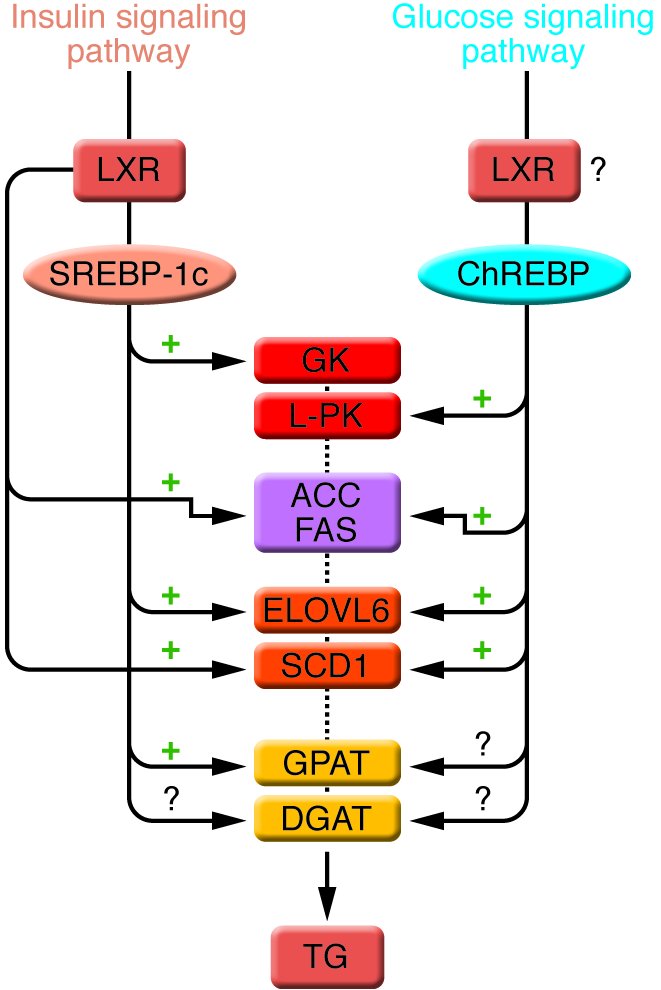
Figure 4
Enzymes of TG synthesis are transcriptionally regulated by ChREBP, SREBP-1c, and LXRs in liver.
Options:
View larger image
(or click on image)
Download as PowerPoint
The conversion of glucose into TGs is nutritionally regulated, and both insulin and glucose signaling pathways are activated in response to dietary carbohydrates to synergistically induce gene expression. The transcription factor SREBP-1c mediates the effect of insulin on GK , ACC , FAS , ELOVL6 , SCD1 , and GPAT . LXRs are also important regulators of TG synthesis, through the direct transcriptional activation of ACC , FAS , and SCD1 but also indirectly via the insulin-mediated transcriptional activation of SREBP1-c. ChREBP, which is induced by glucose, is required for the induction of L-PK , which is exclusively dependent on glucose. The induction of ACC , FAS , ELOVL6 , and SCD1 genes is under the synergistic action of ChREBP and SREBP-1c in response to glucose and insulin, respectively. The direct effect of ChREBP on GPAT expression is not clearly established. ChREBP is also a direct transcriptional target of LXRs, and glucose was recently shown to bind and activate LXRs, thereby indicating that LXRs are part of the glucose signaling pathway. However, in our point of view, the relevance of a potential regulation by LXRs of glucose-sensitive genes needs to be demonstrated in a physiological context. The transcriptional regulation of DGAT in liver is, to our knowledge, still largely unknown.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)