Advertisement
Review
Open Access |  10.1172/JCI195633
10.1172/JCI195633
Revisiting renin-angiotensin-aldosterone system in aging: translational insights from bench to bedside and back
Caglar Cosarderelioglu and Peter M. Abadir
Johns Hopkins University School of Medicine, Division of Geriatric Medicine and Gerontology, Baltimore, Maryland, USA
Address correspondence to: Peter Abadir, Johns Hopkins Bayview Medical Center, 5501 Hopkins Bayview Circle, Suite 1A62, Baltimore, MD 21224. Email: Pabadir1@jhu.edu.
Find articles by Cosarderelioglu, C. in: PubMed | Google Scholar
Johns Hopkins University School of Medicine, Division of Geriatric Medicine and Gerontology, Baltimore, Maryland, USA
Address correspondence to: Peter Abadir, Johns Hopkins Bayview Medical Center, 5501 Hopkins Bayview Circle, Suite 1A62, Baltimore, MD 21224. Email: Pabadir1@jhu.edu.
Find articles by Abadir, P. in: PubMed | Google Scholar
Published November 3, 2025 - More info
J Clin Invest. 2025;135(21):e195633. https://doi.org/10.1172/JCI195633.
© 2025 Cosarderelioglu, et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
The renin-angiotensin-aldosterone system (RAAS) is a central regulator of cardiovascular, renal, and fluid homeostasis. Over the past century, our understanding of RAAS has evolved from a unidimensional circulatory hormone system to a complex network that includes local and intracellular signaling pathways. Aging profoundly impacts this system, influencing both systemic and tissue-specific RAAS activity. While levels of systemic RAAS components, such as plasma renin and aldosterone, decline with age, local RAAS components, particularly the proinflammatory angiotensin (Ang)II/AngII type 1 receptor (AT1R) axis, are upregulated in aging tissues, contributing to vasoconstriction, oxidative stress, inflammation, and fibrosis. Conversely, the protective arms of RAAS, the AngII/AT2R and Ang-(1–7)/Mas receptor pathways, are downregulated. Recent advances in geroscience have further illuminated how RAAS intersects with fundamental aging mechanisms, providing a mechanistic framework for understanding RAAS not only as a driver of age-related disease but also as a modifiable contributor to the aging process itself. In this Review, we summarize the evolution of RAAS biology, examine the molecular and functional consequences of aging on RAAS activity, and discuss the translational relevance of these findings. Finally, we explore emerging therapeutic strategies targeting RAAS components as potential interventions to promote healthy aging and reduce age-related disease burden, emphasizing a translational arc moving from bedside to bench and back, with the ultimate goal of improving patient outcomes.
Aging is accompanied by a broad spectrum of physiological changes that influence cardiovascular, renal, and metabolic function. Among the systems most sensitive to these changes is the renin-angiotensin-aldosterone system (RAAS). Alterations in RAAS with age are increasingly linked to age-associated disorders and geriatric syndromes. This Review examines age-related RAAS changes and their biological and clinical implications in the context of aging.
-
Overview of the RAAS
The RAAS was initially identified as a component of the endocrine system responsible for regulating water and electrolyte balance and systemic vascular resistance. The classical RAAS cascade (detailed in Figure 1) is initiated by release of renin into the bloodstream by the juxtaglomerular cells (JG cells) of the renal afferent arterioles, which store prorenin. Prorenin is cleaved into its active form, renin (1), and released into the bloodstream in response to specific physiological stimuli, including changes in renal BP, variations in sodium chloride concentration, increased sympathetic nervous activity, and feedback from humoral factors such as potassium (1, 2). In a rate-limiting step, renin converts the precursor angiotensinogen into angiotensin I (AngI), which is subsequently converted into AngII by angiotensin-converting enzyme (ACE) (3). AngII acts as the first major bioactive molecule, exerting opposing effects by binding to AngII receptors type 1 and 2 (AT1R and AT2R). Activation of AT1R promotes vasoconstriction, cellular proliferation, growth, and generation of ROS such as superoxide, whereas AT2R activation induces vasodilation and reduces inflammation, oxidative stress, and fibrosis (4). Details on receptors are provided in Table 1.
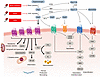 Figure 1
Figure 1Summary of the renin-angiotensin-aldosterone system. As the first and rate-limiting step, renin converts angiotensinogen, a precursor molecule, into the decapeptide AngI. AngI is subsequently converted into AngII by ACE, a zinc metalloprotease predominantly found in the endothelial cells of the lungs (3). Cathepsin and chymase are also capable of hydrolyzing AngI to AngII. AngII exerts opposing effects: vasoconstriction through its binding to AT1R and vasodilation via AT2R. AngII promotes aldosterone production in the adrenal gland zona glomerulosa by enhancing the function of the steroidogenic acute regulatory (StAR) protein and aldosterone synthase, and causes an increase in sodium retention and potassium expulsion, resulting in a rise in water retention and BP. Glutamyl aminopeptidase A (AP-A) cleaves the N-terminal aspartate residue from AngII, producing the heptapeptide AngIII, which is subsequently converted to the hexapeptide AngIV by alanyl aminopeptidase N (AP-N) through cleavage of the N-terminal arginine. AngIV can then be further metabolized into Ang-(3–7) by the action of carboxypeptidase P and prolyl oligopeptidase. Alternatively, AngII can be converted into a heptapeptide Ang-(1–7) by carboxypeptidase P and ACE2, an isoform of ACE (3). ACE2 also catalyzes the conversion of AngI to Ang-(1–9), which can then be converted into Ang-(1–7) by ACE or produced directly from AngI via neutral endopeptidase. A newly identified component of the RAAS is alamandine, which is formed either by decarboxylation of Ang-(1–7) or through ACE2-mediated cleavage of angiotensin A—derived from the decarboxylation of AngII (5). ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; AngI, angiotensin I; AngII, angiotensin II; AngIII, angiotensin III; AngIV, angiotensin IV; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor.
In addition to its systemic endocrine functions, the RAAS also operates via autocrine and paracrine mechanisms within specific tissues. While aldosterone is primarily synthesized in the adrenal cortex, several key components of the RAAS, such as angiotensinogen, renin, ACE, angiotensin receptors, and mineralocorticoid receptor, can be locally synthesized in tissues including the heart, kidneys, and brain (5, 6), referred to as the tissue RAAS. In the intracrine RAS (renin-angiotensin system), the system’s peptide effectors are synthesized and remain entirely within the originating cells. Notably, while angiotensin receptors are classically located on the plasma membrane, they have also been identified in high abundance within intracellular compartments, including the nucleus, mitochondria, and neurosecretory vesicles (7–12). Tissue RAS components can be regulated independently and play specialized roles in organ development, repair, fibrosis, inflammation, and remodeling. AngII, renin, and ACE can be localized to cytoplasm and nuclei, while ACE has been found on the endoplasmic reticulum (ER) (12, 13). Additionally, RAS components were detected in exosomes isolated from blood and urine of patients with hypertension (14).
-
Impact of aging on the RAAS
Numerous studies have demonstrated that systemic RAAS activity diminishes with advancing age. Compared with younger individuals, older adults exhibit lower baseline plasma renin and aldosterone levels (15, 16). Additionally, the responsiveness of renin release to physiological stimuli, such as upright posture, sodium depletion, and hypotension, is markedly blunted in older adults (17, 18). Experimental models in aging animals have also confirmed decreases in both the synthesis and release of renal renin, contributing to the observed decline in circulating renin levels (19, 20). Several mechanisms have been proposed to explain this age-associated suppression of systemic RAAS. These include elevated arterial pressure leading to baroreceptor-mediated inhibition of renin release; reduced β1-adrenergic receptor sensitivity in renal tissues; and in some cases, impaired sympathetic innervation to the JG cells, particularly in individuals with autonomic dysfunction (21, 22). Despite declines in both renin and aldosterone levels with age, the aldosterone-to-renin ratio (ARR) tends to increase (23, 24), which may signal renin-independent aldosterone production. A recent study identified accumulation of aldosterone-producing cell clusters (APCCs) in the adrenal cortex of aging individuals, which may contribute to autonomous aldosterone secretion (25).
Moreover, serum ACE activity has been shown to decline in healthy older men (26). Interestingly, studies in aging animals have shown that renal AngII content increases, despite the overall reduction in circulating RAAS activity. In animal studies, AngII production and AT1R expression and sensitivity are upregulated in various tissues during aging (20, 27, 28). For example, AngII levels were increased in kidney as were ACE, and AT1R levels in hearts and vasculature of aged rats and primates, potentially contributing to structural and functional renal and cardiac remodeling, and suggesting an important role in senescence (28–31). On the other hand, AT2R, a member of RAAS’s protective arm, becomes less effective with age (32–34). Expression of AT2R declines with age in endothelial, neural, and renal tissues, potentially weakening the counterregulatory balance within the RAAS (20, 35, 36).
Furthermore, the protective Ang-(1–7)/Mas receptor axis is also downregulated with aging in mice, rats, and humans (21, 37, 38). Overall, current evidence suggests that while systemic RAAS activity is generally maintained or reduced with age, tissue RAS appears to be regulated differently. Moreover, biological sex influences the effect of aging on RAAS enzyme activity: older men showed significantly lower ACE activity than both older women and younger men (26).
-
RAAS and geroscience
Geroscience has emerged as a critical framework for investigating biological systems affected by aging, including the RAAS. Notably, RAAS signaling intersects with several of the hallmarks of aging, which were recently updated to include two new features: extracellular matrix (ECM) changes and psychosocial isolation (39, 40). This section explores how dysregulation of RAAS may contribute to the biology of aging in the context of these hallmarks (Figure 2).
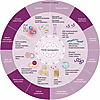 Figure 2
Figure 2Dysregulated renin-angiotensin-aldosterone system and hallmarks of aging. Dysregulation of the renin–angiotensin–aldosterone system (RAAS) can contribute to each of the hallmarks of aging. AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; ECM, extracellular matrix; ER, endoplasmic reticulum; HSC, hematopoietic stem cell; IGF-1, insulin-like growthfactor 1; IL, interleukin; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RAAS, renin-angiotensin-aldosterone system; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; TNF-α, tumor necrosis factor-alpha.
Epigenetic alterations. The RAAS modulates epigenetic regulators such as DNA methyltransferases and histone acetyltransferases, leading to altered gene expression and cellular aging (41–44). Expression of RAAS-related genes is also regulated by epigenetic mechanisms, including DNA methylation, histone modifications, and miRNAs (45–47).
It has been shown that AngII modulates expression of histone demethylase 4A (KDM4A) and activation of class I HDAC1/2 (48). Consistent with this, silencing of HDAC1 or HDAC2 attenuated proliferation and migration of cardiac fibroblasts induced by AngII. Moreover, treatment with an HDAC inhibitor attenuated AngII-induced matrix metalloproteinase-9 and IL-18 expression and ECM production (collagen I, collagen III, and fibronectin) (49). Recent studies have revealed that, beyond stimulating expression of various genes linked to hypertrophy and fibrosis, AngII also influences cellular functions through epigenetic modifications in target cells (50). For example, AngII increases activity of class I HDAC1/2, leading to decreased histone acetylation at H3K9/14 and H4K8, which in turn suppresses Npr1 gene transcription. This downregulation reduces natriuretic peptide receptor-A protein expression and cGMP levels, ultimately impairing renal and vascular reactivity (51). Moreover, AngII promotes recruitment of SET1, a histone H3K4 trimethyltransferase, to the endothelin-1 promoter, and enhanced endothelin-1 expression contributes to persistent arterial hypertension and leads to organ damage, such as cardiac hypertrophy (52, 53).
Loss of proteostasis. Alterations in the RAAS can disrupt cellular protein homeostasis by increasing proteostatic stress and promoting the buildup of damaged or misfolded proteins via excessive activation of AngII signaling (4). This accumulation contributes to cellular dysfunction and accelerates the aging process. Research indicates that diseases associated with AngII share common age-related molecular disturbances such as impaired mitochondrial function and inadequate ER stress responses (54). These impairments foster formation of insoluble protein aggregates, which are worsened by reduced autophagic activity and the spread of cellular senescence (55). AngII contributes to ER stress through multiple pathways. Inhibiting the classical RAAS pathway can mitigate ER stress and inhibition of protein misfolding, while the counterregulatory arm of RAAS may offer protective effects (56, 57). Furthermore, disruptions in proteostasis can drive chronic inflammation by sustaining protein buildup, compounding the detrimental effects on cellular aging (54).
Disabled macroautophagy. The relationship between RAAS and macroautophagy is complex and context dependent. While AngII has been shown to induce autophagy in some cell types, including cardiomyocytes and neuronal cells, its effects vary with receptor subtype and physiological conditions (58). In cardiomyocytes, AT1R activation promotes autophagy, whereas AT2R appears to suppress it (59). Ang-(1–9)/AT2R was found to reduce both basal and AngII-induced autophagy in cardiomyocytes (60). Moreover, in neuronal PC12 cells, AngII triggers autophagy and apoptosis in an AT1R-dependent manner, reversible by the angiotensin receptor blocker (ARB) losartan (61). However, in renal proximal tubules, AngII or aldosterone infusion increases protein aggregates without upregulating autophagy, suggesting impaired proteostasis (54, 62). This aligns with the idea that in certain tissues, RAAS activation may exacerbate proteostatic stress without adequately triggering autophagic clearance. In hepatic stellate cells, AngII-induced ROS led to defective autophagosome clearance and fibrogenesis, and this was mitigated by alamandine, which shifted RAAS signaling toward the protective ACE2/MrgD axis (63). Low-dose AngII has also been shown to activate protective autophagy via ULK1 in renal tubular cells, suggesting a beneficial role in acute settings (64).
The dual nature of autophagy in response to AngII is further illustrated in cardiomyocytes under hypoxic conditions. Short-term AngII exposure may stimulate protective autophagy to mitigate cellular stress; however, with prolonged anoxia, apoptotic mechanisms become dominant. This temporal dynamic suggests that autophagy may serve as an early adaptive mechanism that becomes maladaptive if sustained (65).
Mitochondrial dysfunction. Age-related changes in the expression of mitochondrial RAAS receptors, with a shift toward increased AT1R over AT2R, stimulate NADPH oxidase and result in elevated mitochondrial ROS production. This elevation compromises mitochondrial integrity and function and alters mitochondrial membrane potential, reducing ATP synthesis and amplifying ROS and generation of peroxynitrite, a damaging molecule that disrupts the ETC (12, 66–68). Excess mitochondrial ROS are strongly linked to oxidation of mitochondrial proteins and lipids and to DNA mutations, which can drive cellular senescence and apoptosis (5, 11, 12, 67).
Supporting the detrimental impact of AT1R signaling is the finding that AT1R-knockout mice exhibit an extended lifespan and upregulation of mitochondrial and longevity-associated genes such as Nampt and Sirt3 in the kidney (69, 70). Moreover, chronic activation of the AngII/AT1R/NOX pathway suppresses SIRT3 levels, increasing neuronal susceptibility to oxidative stress, and ARBs mitigate these detrimental effects in aged animal models (71). Intracellular AngII/AT1R/NOX signaling elevates superoxide levels, which uncouple eNOS, reducing NO bioavailability and mitochondrial NOS activity (72). Studies have also shown that ARBs and ACE inhibitors (ACEIs) are effective in reducing AngII-induced mitochondrial dysfunction and its associated deleterious effects (5, 73).
Chronic inflammation. Chronic, low-grade inflammation that increases with age — inflammaging — is a major contributor to the pathogenesis of age-related diseases and syndromes such as frailty (74). The RAAS plays a central role in driving this persistent inflammatory state. AngII promotes the production of proinflammatory cytokines such as IL-6, TNF-α, and IL-17A through activation of AT1R in macrophages and T lymphocytes (75–78). This cytokine release fuels systemic inflammation and contributes to endothelial dysfunction, arterial stiffness, and tissue fibrosis (4, 79). In addition, AngII upregulates adhesion molecules and chemokines, which enhance immune cell recruitment to vascular tissues, amplifying local inflammation and promoting atherosclerosis. Importantly, this is not a unidirectional pathway: inflammatory cytokines such as IL-6 can also stimulate expression of RAAS components such as angiotensinogen and renin, forming a self-sustaining proinflammatory loop (80–83). Furthermore, AngII exposure downregulates protective factors such as Klotho and PGC-1α, both of which decline with age and are essential for suppressing oxidative and inflammatory stress (75). Additionally, AngII modulates epigenetic regulators such as miR-155 and miR-146a that participate in the inflammatory aging phenotype (4, 84).
Counterbalancing this, components of the alternative RAAS axis — particularly through Mas receptor (MasR) and AT2R — exhibit antiinflammatory effects by inhibiting NF-κB signaling and cytokine release. Modulating RAAS signaling, either by inhibiting AT1R or enhancing protective pathways such as ACE2/Ang-(1–7)/Mas, offers a promising approach to mitigate inflammaging and the associated functional decline in multiple tissues.
Cellular senescence. Accumulating evidence indicates that the RAAS plays a role in the induction and progression of senescence, especially in vascular and renal tissues. Chronic, low-dose AngII exposure has been shown to promote senescence in kidney tissues and contribute to the formation of an inflammatory microenvironment through release of senescence-associated secretory phenotype (SASP) factors and recruitment of immune cells (85). Endothelial cells, in particular, appear to be highly susceptible to AngII-induced senescence. In the INK-ATTAC transgenic mouse model, elimination of senescent cells prevented AngII-induced inflammation and tissue damage, highlighting the therapeutic potential of senolytic interventions targeting RAAS-mediated senescence (85).
In vitro studies further demonstrate a biphasic response to AngII (86). While transient AngII exposure enhances endothelial functions such as proliferation, migration, and angiogenesis, prolonged exposure impairs endothelial viability; induces apoptosis; and upregulates senescence markers including senescence-associated β-gal (SA–β-gal) activity, p21, and proinflammatory cytokines. ARBs mitigated these effects in cell culture, suggesting a direct role for the AngII/AT1R axis in endothelial aging and dysfunction (86).
AngII also accelerates senescence in vascular smooth muscle cells (VSMCs), contributing to atherosclerosis development through a p21-dependent mechanism (87). This process involves AT1R-mediated activation of signaling molecules including Ras, MAPKs (such as ERK1/2), and transcription factors like NF-κB and AP-1, leading to oxidative stress and upregulation of cell-cycle inhibitors such as p53 and p21 (88). Notably, these detrimental effects are reversed by the ARB losartan, indicating that RAAS inhibitors (RAASi) may attenuate aging-associated cellular decline (89, 90).
Moreover, in mice, inactivation of AT1R has been associated with a reduction in AngII-induced cellular senescence markers. Mice lacking AT1R exhibited extended lifespan compared with controls, potentially due to reduced oxidative stress and enhanced expression of prosurvival genes. These findings suggest that AT1R plays a pivotal role in regulating senescence and aging at the cellular level (69, 91).
Altered intercellular communication and ECM changes. Progressive disruption of intercellular communication leads to systemic dysfunction and loss of homeostasis (39). The RAAS plays a role in this process, acting as a neurohormonal axis that not only regulates cardiovascular and renal physiology but also may contribute to age-related impairments in cellular signaling. With age, the AngII/AT1R axis becomes overactivated, which exerts proinflammatory, profibrotic, and pro-oxidative effects across multiple tissues, as mentioned above (92). AngII can induce SASP, amplifying local and systemic inflammatory signals that disturb paracrine and endocrine communication (85). Moreover, RAAS interacts with other aging-relevant signaling networks, including insulin/IGF-1, adrenergic, and dopaminergic pathways, contributing to the breakdown of hormetic regulation and adaptive stress responses (93).
Furthermore, RAAS activation contributes to ECM remodeling and fibrosis by promoting collagen deposition and altering matrix turnover (94). These structural changes compromise tissue integrity and elasticity, further accelerating age-related functional decline.
Dysbiosis. Bidirectional interplay between gut microbiota and the RAAS may influence age-related physiological decline and pathology (95). Dysbiosis becomes more prevalent with aging and is increasingly recognized as a contributor to inflammaging, metabolic disorders, and neurodegeneration (96). Under physiological conditions, local gastrointestinal (GI) RAS helps maintain homeostasis in digestion, electrolyte transport, immune modulation, and mucosal protection (95). Studies demonstrate that dysbiosis can alter local RAS activity. Some studies also suggest that microbiota-derived short-chain fatty acids (SCFAs) may modulate local RAS components through SCFA receptors expressed in the renal vasculature (97), indicating a potential bidirectional relationship between gut microbes and the RAS. In contrast, microbial modulation — through fecal microbiota transplantation or prebiotic/probiotic supplementation — can shift RAS activity toward the ACE2/Mas axis, attenuating age-related tissue damage and systemic inflammation, and improving outcomes in animal models of neurological aging (95, 96). The AngII/AT1R axis promotes inflammation and oxidative stress in the gut epithelium, contributing to increased intestinal permeability and barrier dysfunction (98). Conversely, counteracting the effects of AngII has been shown to partially restore microbial diversity, reduce intestinal inflammation, and improve epithelial barrier function (99, 100).
The relationship between additional hallmarks of aging and RAAS is summarized in Table 2.
-
RAAS and lifespan
Both pharmacological interventions and genetic studies have highlighted the influence of RAAS on longevity. Inhibition of ACE homologs in C. elegans and Drosophila and long-term inhibition of AngII signaling in rodents have been associated with lifespan extension (101). For instance, chronic administration of ACEIs or ARBs has been shown to double the lifespan of hypertensive rats (102, 103). Similarly, treatment with the ACEI enalapril in normotensive Wistar rats not only reduced body weight gain but also extended lifespan, suggesting benefits beyond BP control (104). However, these studies did not directly compare RAASi with other antihypertensive drug classes such as calcium channel blockers, which limits conclusions regarding the specificity of RAAS blockade for lifespan extension.
Genetic studies further corroborate these findings. Mice lacking the AT1A receptor (Agtr1a−/− mice) exhibit a 26% increase in lifespan compared with WT counterparts (69). This longevity is accompanied by reduced oxidative stress, diminished organ damage, and upregulation of prosurvival genes, such as Sirt3, Nampt, and Klotho (69).
In humans, polymorphisms in the AGTR1 gene, which encodes AT1R, have been linked to exceptional longevity (105). Notably, the GG genotype of the rs275653 promoter variant was found to be more prevalent among centenarians and is associated with lower AT1R expression and reduced BP (105). These genetic associations suggest that diminished AT1R activity may confer protective effects against age-related pathologies (54).
Furthermore, AngII has been shown to suppress the expression of SIRT1 (106), a key regulator of cellular stress responses and longevity. This suppression exacerbates oxidative stress and promotes cellular senescence. Importantly, elevated circulating levels of AngII have been correlated with higher long-term all-cause mortality, independent of conventional cardiovascular risk factors (107).
Collectively, these findings underscore the pivotal role of the RAAS, particularly the AngII/AT1R axis, in modulating aging and lifespan. Therapeutic strategies targeting this pathway, whether through pharmacological agents such as ACEIs and ARBs or genetic modulation, hold promise for promoting healthy aging and extending lifespan (108).
-
Geriatric syndromes, aging-associated diseases, and RAAS
Excessive activation of the classical RAAS pathway contributes to dysfunction in key organs such as brain, kidneys, vasculature, and skeletal muscle, ultimately promoting geriatric syndromes and conditions such as stroke, HF, and chronic kidney disease (CKD).
Dementia, delirium, and depression. The RAS is increasingly recognized as a player in the pathophysiology of neuropsychiatric disorders, including dementia, delirium, and depression. The AngII/AT1R axis contributes to neurodegeneration and dementia, including Alzheimer’s disease (AD), by promoting chronic inflammation, microglial activation, and oxidative damage — factors that accelerate neuronal injury and synaptic loss (109–111). Elevated AngII levels have been associated with reduced gray matter and hippocampal volume, both critical to memory function (112). Additionally, AngII is reported to increase brain amyloid-β levels via multiple mechanisms, such as increasing amyloid precursor protein mRNA, γ-secretase activity, and presenilin expression (113, 114). The brain RAS’s protective arm becomes less effective with aging due to a decline in AT2R expression (32–34). In aged animal models, this reduction in AT2R was associated with enhanced susceptibility to the effects of AT1R overactivation, including increased oxidative damage, inflammation, and neuronal vulnerability (36). Moreover, AT2R oligomerization triggered by amyloid-β may enhance neurodegeneration (115). In AD patient brain tissue, ACE has been shown to be elevated in the hippocampus, frontal cortex, and caudate nucleus regardless of hypertension, and the levels correlate with AD pathology (116, 117). Similarly, cerebrospinal fluid (CSF) ACE activity was found to be elevated in AD (118).
The AngII/AT1R axis can also contribute to AD via vascular changes such as constriction of cerebral vessels, vascular remodeling, impaired cerebrovascular autoregulation, and endothelial dysfunction (119, 120). In line with this, it has been shown that RAS overactivity is correlated with CSF markers of capillary damage such as elevated CSF-soluble PDGFRβ, indicating pericyte damage, and elevated CSF albumin, indicating blood-brain barrier (BBB) breakdown in AD (118). AngII/AT1R signaling can damage the BBB, increase its permeability, and reduce cerebral blood flow via its proinflammatory and pro-oxidant effects (121). Blockade of AT1R and activation of AT2R reverse hypertension-induced cerebrovascular dysfunction and improve barrier function of endothelial cells and diabetes-associated cerebral endothelial dysfunction (122–124).
AngII has been implicated in disruption of insulin signaling in the brain (125), indicating that RAS activation may also contribute to cognitive decline by altering insulin sensitivity. Finally, the cholinergic system — critical for learning and memory — is negatively influenced by RAAS overactivation (126, 127). AngII reduces acetylcholine release and impairs long-term potentiation (LTP), while activation of the alternative RAS axis, such as Ang-(1–7)/MasR, AngIV /AT4R, and the AngII/AT2R has been shown to enhance memory, synaptic plasticity, and neuroprotection (5, 128–131).
The observation that drugs targeting the RAAS, whether ACEIs, ARBs, or AT2R agonists, preserve memory and attenuate amyloid- and tau-related pathology in animal models, coupled with epidemiological and clinical findings linking RAASi use to a lower incidence and slower progression of AD in humans, provides further evidence that dysregulated angiotensin signaling actively contributes to AD (73, 108, 132–136).
Delirium may also be influenced by RAS activity. Elevated levels of AngII may impair cholinergic signaling and contribute to BBB dysfunction — both of which are factors implicated in delirium pathogenesis (109). Some observational studies suggest a lower incidence of delirium in patients treated with ARBs, though more research is needed (137, 138).
The RAS may influence mood regulation through several mechanisms, such as its effects on neurogenesis and hypothalamic-pituitary-adrenal axis activity (139–141). Increased AngII/AT1R signaling has been linked to elevated cortisol and depressive symptoms. Animal studies and limited clinical data suggest that RAASi may exert antidepressant-like effects, potentially enhancing serotonin and glutathione availability and reducing neuronal damage by decreasing oxidative stress, microglial activation, and levels of inflammatory markers such as TNF-α (141). Overall, RAAS modulation may offer neuroprotective and mood-stabilizing benefits, especially in older adults at risk (136).
Frailty, sarcopenia, and falls. Frailty and sarcopenia are highly prevalent geriatric syndromes characterized by a loss of physiological reserve and increased vulnerability to stressors and by a progressive decline in skeletal muscle mass, strength, and function, respectively (142, 143). These conditions substantially increase the risk of falls, hospitalization, dependency, and mortality in older adults (142–147).
Emerging evidence suggests a mechanistic link between the RAAS and the development and progression of both sarcopenia and frailty, mediated by inflammatory, oxidative, and mitochondrial pathways (20, 148). In animal studies, AngII associated with pronounced skeletal muscle atrophy, characterized by upregulation of the E3 ubiquitin ligases atrogin-1 and MuRF-1, increased proteolysis through the ubiquitin–proteasome system, and elevated NADPH and mitochondria-derived ROS generation (149, 150). Moreover, AngII decreased the number and size of regenerating myofibers and inhibited satellite cell regenerative capacity and muscle regeneration. Similar to AngII, agonistic AT1R autoantibodies are capable of activating the AT1R, and elevated concentrations of these autoantibodies have been associated with increased levels of inflammatory cytokines, reduced grip strength, slower gait speed, and a heightened risk of frailty and falls (151, 152).
In contrast to the catabolic influence of AngII/AT1R, components of the protective RAS arm protect muscle from pathological remodeling and muscle insulin resistance (150). For instance, ACE2-deficient mice exhibited premature muscle weakness, along with indicators of the aging process such as induction of p16INK4a, a senescence-associated gene, and aging-associated changes of myofiber structure, effects that were reversible upon Ang-(1–7) application (153).
Human studies increasingly support the clinical relevance of these mechanisms. The Singapore Longitudinal Ageing Study found that ARB use was associated with a reduction in frailty and age-related loss of muscle mass and strength (154), whereas ACEI use had less-consistent effects in different studies (154, 155). Further studies are needed to clarify the benefits of RAASi in maintaining functional independence.
Pressure ulcers, impaired wound healing, and tissue regeneration. The skin expresses multiple components of the RAAS within the epidermis, dermis, and hair follicles, and RAAS has role in key processes essential for wound healing, including cell migration, proliferation, collagen turnover, inflammatory response, and TGF-β signaling, as well as regulation of stem cell proliferation and differentiation, inflammatory responses, fibrosis and scarring, vascular tone, and even skin tumorigenesis (94, 156, 157).
During the early phase of wound healing, increased vascular permeability facilitates leukocyte infiltration and inflammation. Concurrently, epithelial stem cells and dermal fibroblasts are activated. ACE plays a critical role by converting AngI to AngII, thereby promoting stem cell migration and initiating tissue regeneration. In the later regenerative phase, AngII interacts with AT2R to activate the ERK and STAT1/3 pathways, which enhance fibroblast proliferation and granulation tissue formation. Additionally, RAS signaling stimulates the production of profibrotic molecules, supporting collagen deposition and matrix remodeling during the healing process (94).
Dysregulation of the skin RAS in aging, with increased AT1R and decreased AT2R expression, is implicated in abnormal wound healing (157–159). An imbalance in dermal expression of AT1R and AT2R has been linked to structural deterioration, including epidermal thinning, collagen degradation, disruption of the dermal architecture, and loss of subcutaneous fat in diabetic rats (159).
While excessive AT1R signaling impairs tissue repair by promoting fibrosis and delaying re-epithelialization, AT2R activation appears to facilitate regenerative processes (160). Experimental models demonstrate that topical RAAS modulation — particularly with ARBs such as valsartan — enhances healing outcomes by increasing wound blood flow, collagen deposition, and tissue tensile strength and enhancing re-epithelialization, neovascularization, and formation of organized granulation tissue (157, 161–163). These findings underscore the potential for tissue-targeted RAAS therapies to restore regenerative capacity in compromised healing environments.
Polypharmacy. Polypharmacy, often defined as the use of five or more medications, is common in older adults with multiple chronic conditions and increases the risk of adverse drug events. It is important to note that most research studies are conducted using a single medication, whereas in real-life settings, older adults typically take multiple medications. Medications that inhibit the RAAS are frequently prescribed to manage hypertension, HF, and CKD. However, significant interactions can occur when RAASi are combined with other drugs such as diuretics and NSAIDs, such as increased risk of acute kidney injury (AKI) due to synergistic effects on renal perfusion and function. A study found that combined use of an ACEI or ARB, a diuretic, and an NSAID raised the risk of AKI by 31% (164). Additionally, while each class offers therapeutic benefits, combined use of multiple RAAS-modifying drugs — referred to as dual or triple RAAS blockade — may be associated with an increased risk of adverse effects, and combination of these drugs must be considered more cautiously in older adults (165, 166). Careful medication review, monitoring, and individualized treatment based on risk are essential to minimize adverse outcomes in older adults.
Urinary incontinence. AngII inhibition has been shown to decrease both detrusor overactivity and urethral sphincter tone in animal models, leading to reduced urgency urinary incontinence (UUI) and increased stress urinary incontinence (SUI) (167). Similarly, in a population-based study (National Health and Nutrition Examination Survey [NHANES], 2001–2008), the use of ACEIs or ARBs was associated with a 25%–30% reduction in UUI in men. However, ACEI or ARB use was not linked with any changes in SUI in either men or women. No similar UUI reduction was seen with other antihypertensive drug classes, including diuretics, betablockers, or calcium channel blockers (167).
Sleep disturbances. Nocturnal average renin levels are lower in women than in men and decline with age, though their relationship with sleep appears independent of age and sex (168). Besides nocturnal regulation of renin, poor sleep quality has been linked to elevated plasma aldosterone levels, especially in young and middle-aged men and older women. Aldosterone levels increased progressively with worsening sleep quality, suggesting a potential role of sleep in modulating RAAS activity (169).
Sensory impairments. Recent research suggests that the RAAS may also influence sensory function in aging (170–173). Activation of AT1R and the renin/prorenin receptor (PRR) has been associated with the development of age-related macular degeneration (AMD). Preclinical studies indicate that treatments such as ARBs and ACEIs may help reduce choroidal neovascularization in AMD by suppressing inflammation (174, 175). Higher serum aldosterone levels have been associated with better hearing in older adults, and aldosterone may influence age-related hearing processes by upregulating BCL-2 expression and inhibiting pathways involving BAX and caspases (171, 173).
Stroke. The RAAS plays a central role in the pathophysiology of stroke through both systemic and tissue-specific mechanisms. In addition to its well-established contribution to hypertension, overactivation of the AngII/AT1R axis is one of the most significant modifiable risk factors for stroke, contributing to vasoconstriction, increased sympathetic tone, oxidative stress, inflammation, disruption of the BBB, and endothelial dysfunction, key processes in stroke pathogenesis (176). In the setting of cerebral ischemia, AngII/AT1R activation impairs perfusion to the penumbra, potentially exacerbating tissue damage (177).
In contrast, the alternative RAAS axis counteracts the effects of AngII/AT1R activation, exerting vasodilatory, antiinflammatory, and neuroprotective actions (5). Blockade of the AngII/AT1R axis may therefore confer benefits in two primary domains: the cerebral parenchyma, by modulating inflammation and promoting neuronal survival, and the cerebral vasculature, by restoring perfusion and preserving endothelial function (178). Both preclinical and clinical studies support the neuroprotective potential of RAASi through decreasing activity of AT1R. In rodent models of ischemic stroke, ARBs have been shown to reduce infarct size and improve neurological outcomes (179). In the Heart Outcomes Prevention Evaluation (HOPE) trial, the ACEI ramipril significantly reduced stroke risk, with effects likely attributable to RAAS modulation beyond BP control. The Microalbuminuria, Cardiovascular and Renal Outcomes–HOPE (MICRO-HOPE) substudy corroborated these findings, demonstrating greater cardiovascular protection than expected from BP reduction alone (180). Similarly, the Losartan Intervention for Endpoint Reduction (LIFE) trial reported that losartan was more effective than atenolol in stroke prevention, despite comparable antihypertensive efficacy (181). Observational data further suggest that RAASi are associated with reduced in-hospital mortality among patients with acute ischemic stroke (182). However, a large meta-analysis of 147 randomized trials by Wald and Law indicated that most of the stroke risk reduction associated with ACEIs and ARBs may be explained by their BP-lowering effects rather than BP-independent mechanisms (183).
Recent attention has turned to the RAAS-protective arm. Emerging therapeutic strategies targeting these alternative axes — such as AT2R agonists (e.g., C21, CGP42112), Ang-(1–7), and β-arrestin-biased AT1R agonists — are under active investigation for their potential to provide neuroprotection without the deleterious effects associated with classical AT1R stimulation (178).
Atrial fibrillation and heart failure. With advancing age, the dysregulated RAAS contributes substantially to the development and progression of atrial fibrillation (AF) and heart failure (HF), two highly prevalent and interlinked conditions in older adults. Overactivation of the AngII/AT1R axis promotes adverse structural, electrical, and neurohormonal remodeling through inflammation, oxidative stress, and fibrosis and accelerates cardiovascular aging via mechanisms such as mTOR activation and suppression of aging-protective molecules such as sirtuins (e.g., SIRT1), Klotho, and PGC-1α (54, 184). The RAAS stimulates fibroblast proliferation and ECM deposition via upregulation of MAPK signaling and reduced collagenase activity, contributing to atrial structural remodeling and electrophysiological disturbances that promote reentry circuits, involving a reduction in the atrial effective refractory period and shortening of action potential duration (185). Aldosterone further exacerbates this process by activating mineralocorticoid receptors, leading to left atrial dilation and fibrosis, particularly in patients with primary aldosteronism (186).
Similarly, in HF, persistent RAAS activation drives maladaptive remodeling. The AngII/AT1R axis promotes cardiomyocyte hypertrophy, apoptosis, interstitial fibrosis, vascular inflammation, and myocardial stiffening (187–189). AngII has also been shown to increase exosome release from cardiac fibroblasts, and these exosomes upregulate renin, angiotensinogen, AT1R, and AT2R, while downregulating ACE2 and enhancing AngII production, ultimately promoting hypertrophy (190). These changes contribute to both systolic and diastolic dysfunction and increase susceptibility to arrhythmias.
AF and HF frequently coexist and exacerbate one another in older adults, with the bidirectional relationship intensified by RAAS-mediated remodeling. HF increases left atrial pressure and volume, promoting atrial stretch and fibrosis, while AF contributes to reduced cardiac output and tachycardia-induced cardiomyopathy (191, 192). RAAS inhibition represents a key therapeutic strategy to break this pathological loop.
Treatment with ACEI and ARBs has been shown to attenuate cardiac remodeling by limiting left atrial enlargement, dysfunction, and fibrosis and shortening the atrial effective refractory period — changes that are expected to lower the risk of AF in animal models of HF (193). Modulation of RAAS with ACEIs, ARBs, and mineralocorticoid receptor antagonists (MRAs) was shown to reduce the incidence of new-onset and recurrent AF, especially in patients with hypertension or left ventricular dysfunction (194, 195). Importantly, the benefits of RAAS inhibition extend beyond hemodynamic control (196). In both AF and HF, ACEIs and ARBs exert antiinflammatory, antifibrotic, and antioxidative effects. Growing understanding of tissue-specific RAAS adds nuance, particularly in aging tissues where the protective ACE2/Ang-(1–7)/Mas and AT2R axes are suppressed. The balance between the deleterious AngII/AT1R axis and the protective ACE2/Ang-(1–7)/MasR and AT2R pathways is crucial in determining the outcome in AF and HF. Therapeutic strategies aimed at enhancing the protective arm are currently under investigation and may represent the next frontier in RAAS-targeted therapy (197, 198).
Moreover, the Randomized Aldactone Evaluation Study (RALES), Eplerenone Post-AMI Heart Failure Efficacy and Survival Study (EPHESUS), and Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trials established that steroidal MRAs such as spironolactone and eplerenone significantly reduce mortality and hospitalizations in patients with HF with reduced ejection fraction (HFrEF), leading to their strong endorsement in international guidelines (199–201). However, spironolactone did not improve the primary composite outcome of HF hospitalization, resuscitated cardiac arrest, or cardiovascular death in patients with preserved ejection fraction (HFpEF) in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial (202). More recently, the Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients With Heart Failure (FINEARTS-HF) trial and a meta-analysis by Jhund et al. showed that nonsteroidal MRAs led to cardiovascular benefits in patients with mildly reduced ejection fraction (HFmrEF) or HFpEF (203, 204).
CKD. With advancing age, both circulating and intrarenal components of the RAAS undergo marked shifts that predispose the aged kidney to functional decline and structural injury. Plasma renin and aldosterone levels fall, whether at baseline or in response to classical stimuli, and older individuals consequently lose much of their capacity for sodium conservation and potassium excretion, placing them at risk for salt wasting hyponatremia and hyperkalemia (205).
Healthy kidneys predominantly convert AngI to the vasodilator Ang-(1–7) via neprilysin, with a fraction shunted to AngII by ACE. In CKD, this balance reverses: Ang-(1–7) formation falls, while AngII rises, partly because local generation of AngII shifts from ACE toward chymase, an enzyme unleashed by tissue injury (206, 207).
These RAAS alterations have profound hemodynamic and structural consequences. Aged kidneys commonly exhibit afferent arteriolar hyalinization, exaggerated glomerular hypertension, and accelerating sclerotic changes (16, 205). In animal models, mesangial expansion, interstitial fibrosis, and proteinuria can be ameliorated by ACEIs or ARBs (16, 208, 209). In patients with mild or moderate CKD, ARBs and ACEIs reduce BP, slow estimated glomerular filtration rate (eGFR) decline, reduce proteinuria, and delay progression to advanced CKD; however, the effects of ARBs and ACEIs on mortality and cardiovascular risk in patients with CKD remain controversial (210, 211). A recent study showed that aging does not modify RAASi’s beneficial effects on CKD outcomes or their potential adverse effects (211).
Therapeutic modulation of RAAS in aging. As discussed above, the RAAS is increasingly recognized as an important therapeutic target in age-related conditions, given its involvement in regulating vascular tone, inflammation, oxidative stress, and tissue remodeling. In this context, repurposing widely used RAAS-modifying drugs for aging-related indications is an area of growing interest. Table 3 presents an overview of the major therapeutic classes and their mechanisms of action.
-
Future directions and unanswered questions
RAAS, once seen mainly as a fluid and BP regulator, is now recognized as a key player in aging and age-related diseases. It intersects with several aging hallmarks, including inflammation and mitochondrial dysfunction, positioning it as a promising target for therapies aimed at extending lifespan and health span. However, despite advances in understanding RAAS in aging, critical gaps remain. Longitudinal studies are needed to define when and how RAAS shifts occur, and systemic biomarkers poorly reflect local tissue-specific activity, which remains underexplored due to limited measurement tools. The low specificity of antibodies for AT1R and AT2R limits accurate receptor profiling in experimental contexts (212). Additionally, the role of RAAS-targeting autoantibodies in aging, disease progression, and treatment resistance needs further investigation. Sex differences in RAAS activity also remain poorly characterized, despite hormonal influences that may affect disease risk and treatment outcomes. While RAASi are widely used in older adults, their effects on healthy aging, frailty, and cognition remain unclear. Furthermore, the development of antisenescent strategies brings unique clinical challenges. These include identifying optimal windows for intervention, selecting end points that meaningfully reflect functional aging outcomes, and determining appropriate trial durations in populations with heterogenous health trajectories. Clinical trial design must also consider comorbidities that accelerate biological aging and influence treatment responsiveness. Addressing these questions will be vital for the responsible translation of antisenescent medicines targeting RAAS and beyond. Future research should also focus on personalized approaches using biomarkers, autoantibody profiling, and genetic polymorphisms to optimize RAAS-targeted therapies from both the preventative and treatment perspectives in aging populations.
-
Acknowledgments
The author(s) declare that financial support was received for the research and/or publication of this article. The authors are supported by funding from the Johns Hopkins Pepper Older Americans Independence Center, American Geriatrics Society (AGS) National Institute on Aging (NIA) 2R13AG054139-06 (to PMA), NIA career development award K24AG088484 (to PMA) and the Johns Hopkins Artificial Intelligence and Technology Collaboratory P30AG073104 (NIA/NIH) (to PMA).
Address correspondence to: Peter Abadir, Johns Hopkins Bayview Medical Center, 5501 Hopkins Bayview Circle, Suite 1A62, Baltimore, MD 21224. Email: Pabadir1@jhu.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Cosarderelioglu et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(21):e195633. https://doi.org/10.1172/JCI195633.
-
References
- Fountain JH, et al. Physiology, renin angiotensin system. StatPearls. StatPearls Publishing; 2025.
- Kanugula AK, et al. Renin-angiotensin system: updated understanding and role in physiological and pathophysiological states. Cureus. 2023;15(6):e40725.
- Wright JW, Harding JW. The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch. 2013;465(1):133–151.
- Forrester SJ, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738.
- Cosarderelioglu C, et al. Brain renin-angiotensin system at the intersect of physical and cognitive frailty. Front Neurosci. 2020;14:10.3389/fnins.2020.586314.
- Epstein M. Aldosterone and mineralocorticoid receptor signaling as determinants of cardiovascular and renal injury: an extraordinary paradigm shift. Kidney Int Suppl (2011). 2022;12(1):1–6.View this article via: CrossRef Google Scholar
- Vila-Porcile E, Corvol P. Angiotensinogen, prorenin, and renin are co-localized in the secretory granules of all glandular cells of the rat anterior pituitary: an immunoultrastructural study. J Histochem Cytochem. 1998;46(3):301–311.
- Sadoshima J, et al. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75(5):977–984.
- Sherrod M, et al. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):539–546.
- Peters J. Secretory and cytosolic (pro)renin in kidney, heart, and adrenal gland. J Mol Med (Berl). 2008;86(6):711–714.
- Abadir PM, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108(36):14849–14854.
- Abadir PM, et al. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012;38(2):437–445.
- Shen XZ, et al. Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J Biol Chem. 2008;283(15):9957–9965.
- Ahmad S, et al. Chymase in plasma and urine extracellular vesicles: novel biomarkers for primary hypertension [preprint]. https://doi.org/10.1101/2023.11.09.23298324 Posted on medRxiv November 10, 2023.
- Noth RH, et al. Age and the renin-aldosterone system. Arch Intern Med. 1977;137(10):1414–1417.
- Yoon HE, Choi BS. The renin-angiotensin system and aging in the kidney. Korean J Intern Med. 2014;29(3):291–295.
- Weidmann P, et al. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. 1975;8(5):325–333.
- Mulkerrin E, et al. Aldosterone responses to hyperkalemia in healthy elderly humans. J Am Soc Nephrol. 1995;6(5):1459–1462.
- Jung FF, et al. Down-regulation of the intrarenal renin-angiotensin system in the aging rat. J Am Soc Nephrol. 1995;5(8):1573–1580.
- Abadir PM. The frail renin-angiotensin system. Clin Geriatr Med. 2011;27(1):53–65.
- Miller AJ, Arnold AC. The renin-angiotensin system and cardiovascular autonomic control in aging. Peptides. 2022;150:170733.
- Diz DI. Lewis K. Dahl memorial lecture: the renin-angiotensin system and aging. Hypertension. 2008;52(1):37–43.
- Nakama C, et al. The influence of aging on the diagnosis of primary aldosteronism. Hypertens Res. 2014;37(12):1062–1067.
- Peng N, et al. Effect of age on aldosterone-renin ratio in screening primary aldosteronism. J Clin Hypertens (Greenwich). 2025;27(3):e70014.
- Nanba K, et al. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347–355.
- Fernández-Atucha A, et al. Sex differences in the aging pattern of renin–angiotensin system serum peptidases. Biol Sex Differ. 2017;8:5.
- Rubio-Ruíz ME, et al. Angiotensin II and 1-7 during aging in Metabolic Syndrome rats. Expression of AT1, AT2 and Mas receptors in abdominal white adipose tissue. Peptides. 2014;57:101–108.
- Thompson MM, et al. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):1787–1794.
- Diz DI, et al. Aging and the brain renin–angiotensin system: relevance to age-related decline in cardiac function. Future Cardiol. 2025;4(3):237–245.
- Wang M, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41(6):1308–1316.
- Heymes C, et al. Cardiac senescence is associated with enhanced expression of angiotensin II receptor subtypes. Endocrinology. 1998;139(5):2579–2587.
- Lu J, et al. Angiotensin AT2 receptor stimulation inhibits activation of NADPH oxidase and ameliorates oxidative stress in rotenone model of Parkinson’s disease in CATH.a cells. Neurotoxicol Teratol. 2015;47:16–24.
- Villar-Cheda B, et al. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: implications for progression of Parkinson’s disease. Eur J Neurosci. 2010;32(10):1695–1706.
- Villar-Cheda B, et al. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol Aging. 2012;33(1):204.e1–204.11.
- Carey RM. Angiotensin receptors and aging. Hypertension. 2007;50(1):33–34.
- Labandeira-Garcia JL, et al. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129.
- Costa-Besada MA, et al. Paracrine and intracrine angiotensin 1-7/Mas receptor axis in the substantia nigra of rodents, monkeys, and humans. Mol Neurobiol. 2018;55(7):5847–5867.
- Vargas-Castillo A, et al. Angiotensin-(1-7) induces beige fat thermogenesis through the Mas receptor. Metabolism. 2020;103(1–7):154048.
- López-Otín C, et al. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–278.
- Kroemer G, et al. From geroscience to precision geromedicine: Understanding and managing aging. Cell. 2025;188(8):2043–2062.
- Qiu Y, et al. Epigenetic modifications and emerging therapeutic targets in cardiovascular aging and diseases. Pharmacol Res. 2025;211:107546.
- Takeda Y, et al. Epigenetic regulation of the renin-angiotensin-aldosterone system in hypertension. Int J Mol Sci. 2024;25(15):8099.
- Das S, et al. Regulation of angiotensin II actions by enhancers and super-enhancers in vascular smooth muscle cells. Nat Commun. 2017;8(1):1467.
- Ji Y, et al. Roles and mechanisms of histone methylation in vascular aging and related diseases. Clin Epigenetics. 2025;17(1):35.
- Pandey KN. Genetic and epigenetic mechanisms regulating blood pressure and kidney dysfunction. Hypertension. 2024;81(7):1424–1437.
- Stoll S, et al. DNA methylation and histone modification in hypertension. Int J Mol Sci. 2018;19(4):1174.
- Jiang S, Guo Y. Epigenetic clock: DNA methylation in aging. Stem Cells Int. 2020;2020(1):1047896.
- Rosales W, et al. Role of histone demethylases in cardiomyocytes induced to hypertrophy. Biomed Res Int. 2016;2016(1):2634976.
- Somanna NK, et al. Histone deacetyltransferase inhibitors Trichostatin A and Mocetinostat differentially regulate MMP9, IL-18 and RECK expression, and attenuate Angiotensin II-induced cardiac fibroblast migration and proliferation. Hypertens Res. 2016;39(10):709–716.
- Segers VFM, et al. Epigenetic regulation of intercellular communication in the heart. Am J Physiol Heart Circ Physiol. 2019;316(6):H1417–H1425.
- Arise KK, et al. Angiotensin II represses Npr1 expression and receptor function by recruitment of transcription factors CREB and HSF-4a and activation of HDACs. Sci Rep. 2020;10(1):4337.
- Virdis A, et al. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: role of perivascular adipose tissue. Eur Heart J. 2015;36(13):784–794.
- Mengozzi A, et al. Epigenetic signatures in arterial hypertension: focus on the microvasculature. Int J Mol Sci. 2023;24(5):4854.
- Cooper HA, et al. Angiotensin II- and Alzheimer-type cardiovascular aging. Circ Res. 2018;123(6):651–653.
- Rodríguez-Lara SQ, et al. The renin-angiotensin-aldosterone system as a therapeutic target in late injury caused by ischemia-reperfusion. Int J Endocrinol. 2018;2018:3614303.
- Sepúlveda-Fragoso V, et al. Crosstalk between the renin-angiotensin system and the endoplasmic reticulum stress in the cardiovascular system: Lessons learned so far. Life Sci. 2021;284:119919.
- Bild W, et al. Impact of the renin-angiotensin system on the pathogeny and pharmacotherapeutics of neurodegenerative diseases. Biomolecules. 2022;12(10):1429.
- Steckelings UM, Unger T. Angiotensin receptors and autophagy: live and let die. Hypertension. 2009;53(6):898–899.
- Porrello ER, et al. Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension. 2009;53(6):1032–1040.
- Bustamante M, et al. Autophagy fine-tuning by angiotensin-(1-9) in cultured rat cardiomyocytes. Front Cardiovasc Med. 2025;12(1-9):1408325.
- Tian M, et al. Angiotensin II triggers autophagy and apoptosis in PC12 cell line: An in vitro Alzheimer’s disease model. Brain Res. 2019;1718:46–52.
- Cheema MU, et al. Aldosterone and angiotensin II induce protein aggregation in renal proximal tubules. Physiol Rep. 2013;1(4):e00064.
- Huang Y, et al. Alamandine attenuates hepatic fibrosis by regulating autophagy induced by NOX4-dependent ROS. Clin Sci (Lond). 2020;134(7):853–869.
- Sugawara H, et al. Activation of the angiotensin II receptor promotes autophagy in renal proximal tubular cells and affords protection from ischemia/reperfusion injury. J Pharmacol Sci. 2021;145(2):187–197.
- Wang X, et al. Regulation of autophagy and apoptosis in response to angiotensin II in HL-1 cardiomyocytes. Biochem Biophys Res Commun. 2013;440(4):696–700.
- Sastre J, et al. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49(5):427–435.
- Vajapey R, et al. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol. 2014;5:439.
- Toth DD, et al. Angiotensin II alters mitochondrial membrane potential and lipid metabolism in rat colonic epithelial cells. Biomolecules. 2024;14(8):974.
- Benigni A, et al. Disruption of the AngII type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119(3):524–530.
- Benigni A, et al. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257.
- Diaz-Ruiz C, et al. Aging-related overactivity of the angiotensin/AT1 axis decreases sirtuin 3 levels in the substantia nigra, which induces vulnerability to oxidative stress and neurodegeneration. J Gerontol A Biol Sci Med Sci. 2020;75(3):416–424.
- Valdez LB, et al. Heart mitochondrial nitric oxide synthase. Effects of hypoxia and aging. Mol Aspects Med. 2004;25(1–2):49–59.
- Saleh N, et al. Losartan mitigates oxidative stress in the brains of aged and inflamed IL10-/- mice. J Gerontol A Biol Sci Med Sci. 2022;77(9):1784–1788.
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522.
- Cantero-Navarro E, et al. Renin-angiotensin system and inflammation update. Mol Cell Endocrinol. 2021;529:111254.
- Basile DP, et al. Contribution of Th17 cells to tissue injury in hypertension. Curr Opin Nephrol Hypertens. 2021;30(2):151–158.
- Satou R, et al. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr Hypertens Rep. 2018;20(12):100.
- Awad K, et al. Effect of the renin-angiotensin system inhibitors on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2022;97(10):1808–1823.
- Kawai T, et al. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017;125(pt a):4–13.
- dos Passos RR, et al. Immunomodulatory activity of cytokines in hypertension: a vascular perspective. Hypertension. 2024;81(7):1411–1423.
- Luther JM, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006;48(6):1050–1057.
- Thangaraj SS, et al. The mineralocorticoid receptor blocker spironolactone lowers plasma interferon-γ and interleukin-6 in patients with type 2 diabetes and treatment-resistant hypertension. J Hypertens. 2022;40(1):153–162.
- Brasier AR, et al. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22(8):1257–1266.
- Pacurari M, Tchounwou PB. Role of MicroRNAs in renin-angiotensin-aldosterone system-mediated cardiovascular inflammation and remodeling. Int J Inflam. 2015;2015:101527.
- Khan I, et al. Low dose chronic angiotensin II induces selective senescence of kidney endothelial cells. Front Cell Dev Biol. 2021;9:782841.
- Li R, et al. Long-term stimulation of angiotensin II induced endothelial senescence and dysfunction. Exp Gerontol. 2019;119:212–220.
- Kunieda T, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114(9):953–960.
- Min LJ, et al. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8(2):113–121.
- Feng X, et al. Change of telomere length in angiotensin II-induced human glomerular mesangial cell senescence and the protective role of losartan. Mol Med Rep. 2011;4(2):255–260.
- Conti S, et al. Aging and the renin-angiotensin system. Hypertension. 2012;60(4):878–883.
- Mattson MP, Maudsley S. Live longer sans the AT1A receptor. Cell Metab. 2009;9(5):403–405.
- Yi W, et al. Role of angiotensin II in aging. Front Aging Neurosci. 2022;14:1002138.
- Rodriguez-Perez AI, et al. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. Oncotarget. 2016;7(21):30049–30067.
- Qoreishi SH, et al. Exploring the molecular underpinnings of skin regeneration and wound healing: the role of renin angiotensin. Avicenna J Med Biotechnol. 2024;16(3):146–155.
- Jaworska K, et al. Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol Gastrointest Liver Physiol. 2021;321(4):355–366.
- Holmes A, et al. Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl Res. 2020;226:39–56.
- Lu C, et al. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin. 2020;41(8):1111–1118.
- Gao Z-Y, et al. Inhibition of angiotensin II type 1 receptor reduces oxidative stress damage to the intestinal barrier in severe acute pancreatitis. Kaohsiung J Med Sci. 2023;39(8):824–833.
- Chittimalli K, et al. Restoration of the gut barrier integrity and restructuring of the gut microbiome in aging by angiotensin-(1-7). Clin Sci (Lond). 2023;137(11):913–930.
- Robles-Vera I, et al. Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br J Pharmacol. 2020;177(9):2006–2023.
- Egan BM, et al. Control of aging by the renin–angiotensin system: a review of C. elegans, Drosophila, and mammals. Front Pharmacol. 2022;13:938650.
- Linz W, et al. Long-term ACE inhibition doubles lifespan of hypertensive rats. Circulation. 1997;96(9):3164–3172.
- Linz W, et al. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000;35(4):908–913.
- Santos EL, et al. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem Pharmacol. 2009;78(8):951–958.
- Benigni A, et al. Variations of the angiotensin II type 1 receptor gene are associated with extreme human longevity. Age (Dordr). 2013;35(3):993–1005.
- Li Y, et al. Angiotensin II induces mitochondrial oxidative stress and mtDNA damage in osteoblasts by inhibiting SIRT1–FoxO3a–MnSOD pathway. Biochem Biophys Res Commun. 2014;455(1–2):113–118.
- Jia E-Z, et al. Relationship of renin-angiotensin-aldosterone system polymorphisms and phenotypes to mortality in Chinese coronary atherosclerosis patients. Sci Rep. 2014;4(1):4600.
- de Cavanagh EMV, et al. Renin-angiotensin system inhibitors positively impact on multiple aging regulatory pathways: Could they be used to protect against human aging? Physiol Rep. 2024;12(12):e16094.
- Jackson L, et al. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19(3):876.
- Farag E, et al. The renin angiotensin system and the brain: New developments. J Clin Neurosci. 2017;46:1–8.
- Cosarderelioglu C, et al. Higher angiotensin II type 1 receptor levels and activity in the postmortem brains of older persons with Alzheimer’s dementia. J Gerontol A Biol Sci Med Sci. 2021;77(4):664–672.
- S Y, et al. Angiotensin II blood levels are associated with smaller hippocampal and cortical volumes in cognitively normal older adults. J Alzheimers Dis. 2020;75(2):521–529.
- Zhu D, et al. Central angiotensin II stimulation promotes β amyloid production in Sprague Dawley rats. PLoS One. 2011;6(1):e16037.
- Gebre AK, et al. Targeting renin–angiotensin system against Alzheimer’s Disease. Front Pharmacol. 2018;9:440.
- AbdAlla S, et al. Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J Biol Chem. 2009;284(10):6554–6565.
- Miners JS, et al. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer’s disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34(2):181–193.
- Miners S, et al. Angiotensin-converting enzyme levels and activity in Alzheimer’s disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res. 2009;1(2):163–177.View this article via: PubMed Google Scholar
- Kehoe PG, et al. Cerebrospinal fluid changes in the renin-angiotensin system in Alzheimer’s disease. J Alzheimers Dis. 2019;72(2):525–535.
- Pires PW, et al. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304(12):1598–1614.
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7(6):476–484.
- Miners JS, et al. ACE variants and association with brain Aβ levels in Alzheimer’s disease. Am J Transl Res. 2011;3(1):73–80.View this article via: PubMed Google Scholar
- Gallego-Delgado J, et al. Angiotensin receptors and β-catenin regulate brain endothelial integrity in malaria. J Clin Invest. 2016;126(10):4016–4029.
- Alhusban A, et al. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344(2):348–359.
- Fouda AY, et al. Brain vasculature and cognition. Arterioscler Thromb Vasc Biol. 2019;39(4):593–602.
- Folli F, et al. Crosstalk between insulin and angiotensin II signalling systems. Exp Clin Endocrinol Diabetes. 1999;107(2):133–139.
- Bartus RT, et al. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414.
- Kehoe PG. The coming of age of the angiotensin hypothesis in Alzheimer’s disease: progress toward disease prevention and treatment? J Alzheimers Dis. 2018;62(3):1443–1466.
- Lee J, et al. Potentiation of cholinergic transmission in the rat hippocampus by angiotensin IV and LVV-hemorphin-7. Neuropharmacology. 2001;40(4):618–623.
- Lew RA, et al. Angiotensin AT4 ligands are potent, competitive inhibitors of insulin regulated aminopeptidase (IRAP). J Neurochem. 2003;86(2):344–350.
- Stragier B, et al. Metabolism of angiotensin II is required for its in vivo effect on dopamine release in the striatum of the rat. J Neurochem. 2004;90(5):1251–1257.
- Davis CJ, et al. AT4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-D-aspartate dependent form of long-term potentiation. Neuroscience. 2006;137(4):1369–1379.
- Cosarderelioglu C, et al. Angiotensin receptor blocker use is associated with upregulation of the memory-protective angiotensin type 4 receptor (AT4R) in the postmortem brains of individuals without cognitive impairment. Geroscience. 2023;45(1):371–384.
- Yasar S, et al. Antihypertensive drugs decrease risk of Alzheimer disease: ginkgo evaluation of memory study. Neurology. 2013;81(10):896–903.
- Ouk M, et al. The use of angiotensin-converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer’s disease: the importance of blood-brain barrier penetration and APOE ε4 carrier status. Alzheimers Res Ther. 2021;13(1):43.
- Loera-Valencia R, et al. Brain renin-angiotensin system as novel and potential therapeutic target for Alzheimer’s disease. Int J Mol Sci. 2021;22(18):10139.
- Yasmin S, et al. The role of ACE inhibitors and ARBs in preserving cognitive function via hypertension Management: A critical Update. Brain Res. 2025;1850:149400.
- Farag E, et al. Association between use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and postoperative delirium. Anesthesiology. 2020;133(1):119–132.
- Chen G, et al. Influence of renin-angiotensin system inhibitors on postoperative delirium in patients with pulmonary arterial hypertension: a secondary analysis of a retrospective cohort study. Front Psychiatry. 2022;13:851104.
- Bordet S, et al. An open-label, non-randomized, drug-repurposing study to explore the clinical effects of angiotensin II type 1 (AT1) receptor antagonists on anxiety and depression in Parkinson’s Disease. Mov Disord Clin Pract. 2025;12(5):653–658.
- Gong S, Deng F. Renin-angiotensin system: The underlying mechanisms and promising therapeutical target for depression and anxiety. Front Immunol. 2023;13:1053136.
- Vian J, et al. The renin-angiotensin system: a possible new target for depression. BMC Med. 2017;15(1):144.
- Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156.
- Cruz-Jentoft AJ, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Aging. 2019;48(4):601.
- Fried LP, et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1(1):36–46.
- Beaudart C, et al. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLos One. 2019;12(1):e0169548.
- Woo J, et al. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2019;16(3):247–252.
- Dao T, et al. Sarcopenia and muscle aging: a brief overview. Endocrinol Metab (Seoul). 2020;35(4):716–732.
- Ekiz T, et al. Rewinding sarcopenia: a narrative review on the renin-angiotensin system. Aging Clin Exp Res. 2021;33(9):2379–2392.
- Sukhanov S, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342(2):143–147.
- Saravi B, et al. The tissue renin-angiotensin system and its role in the pathogenesis of major human diseases: quo vadis? Cells. 2021;10(3):650.
- Abadir PM, et al. Discovery and validation of agonistic angiotensin receptor autoantibodies as biomarkers of adverse outcomes. Circulation. 2017;135(5):449–459.
- Herse F, LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am J Reprod Immunol. 2013;69(4):413–418.
- Takeshita H, et al. Angiotensin-converting enzyme 2 deficiency accelerates and angiotensin 1-7 restores age-related muscle weakness in mice. J Cachexia Sarcopenia Muscle. 2018;9(5):975–986.
- Ng TP, et al. Angiotensin receptor blockers use and changes in frailty, muscle mass, and function indexes: Singapore Longitudinal Ageing Study. JCSM Rapid Commun. 2021;4(2):111–121.View this article via: CrossRef Google Scholar
- Veronese N, et al. Angiotensin-converting enzyme inhibitor use and incident frailty: a longitudinal cohort study. Drug Aging. 2019;36(4):387–393.
- Aleksiejczuk M, et al. The expression of the renin-angiotensin-aldosterone system in the skin and its effects on skin physiology and pathophysiology. J Physiol Pharmacol. 2019;70(3):325–336.
- Abadir P, et al. Topical reformulation of valsartan for treatment of chronic diabetic wounds. J Invest Dermatol. 2018;138(2):434–443.
- Cooper ME. The role of the renin-angiotensin-aldosterone system in diabetes and its vascular complications. Am J Hypertens. 2004;17(11 pt 2):16S–20S.
- Hao SY, et al. Activation of skin renin-angiotensin system in diabetic rats. Endocrine. 2011;39(3):242–250.
- Faghih M, et al. Knockout of Angiotensin AT2 receptors accelerates healing but impairs quality. Aging (Albany NY). 2015;7(12):1185–1197.
- Kamber M, et al. Angiotensin II inhibitor facilitates epidermal wound regeneration in diabetic mice. Front Physiol. 2015;6:170.
- Nidadavolu LS, et al. Valsartan nano-filaments alter mitochondrial energetics and promote faster healing in diabetic rat wounds. Wound Repair Regen. 2021;29(6):927–937.
- Fang Q-Q, et al. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-β1 pathways. Sci Rep. 2018;8(1):3332.
- Lapi F, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525.
- Chilton RJ, Silva-Cardoso J. Mineralocorticoid receptor antagonists in cardiovascular translational biology. Cardiovasc Endocrinol Metab. 2023;12(3):e0289.
- Whitlock R, et al. The association between dual RAAS inhibition and risk of acute kidney injury and hyperkalemia in patients with diabetic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2023;38(11):2503–2516.
- Elliott CS, Comiter CV. The effect of angiotensin inhibition on urinary incontinence: data from the National Health and Nutrition Examination Survey (2001-2008). Neurourol Urodyn. 2014;33(8):1178–1181.
- Schüssler P, et al. Sleep and active renin levels — interaction with age, gender, growth hormone and cortisol. Neuropsychobiology. 2010;61(3):113–121.
- Li X, et al. Poor sleep quality was associated with increased plasma aldosterone concentration in community dwellers, a cross-sectional study. Sci Rep. 2025;15(1):10817.
- Marin Garcia PJ, Marin-Castaño ME. Angiotensin II-related hypertension and eye diseases. World J Cardiol. 2014;6(9):968–984.
- Frisina RD, et al. Age-related hearing loss: prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging (Albany NY). 2016;8(9):2081–2099.
- Förster CY, et al. Interplay between noise-induced sensorineural hearing loss and hypertension: pathophysiological mechanisms and therapeutic prospects. Front Cell Neurosci. 2025;19:1523149.
- Tadros SF, et al. Higher serum aldosterone correlates with lower hearing thresholds: a possible protective hormone against presbycusis. Hear Res. 2005;209(1-2):10–18.
- Holappa M, et al. Many faces of renin-angiotensin system – focus on eye. Open Opthalmol J. 2017;11:122–142.View this article via: CrossRef Google Scholar
- Ren C, et al. Renin-angiotensin system inhibitor usage and age-related macular degeneration among hypertensive patients: results from the national health and nutrition examination survey, 2005-2008. J Ophthalmol. 2020;2020:4252031.
- De Silva TM, et al. Activation of the central renin-angiotensin system causes local cerebrovascular dysfunction. Stroke. 2021;52(7):2404–2413.
- Walther T, et al. Ischemic injury in experimental stroke depends on angiotensin II. FASEB J. 2002;16(2):169–176.
- Andone S, et al. Neuroprotection in stroke-focus on the renin-angiotensin system: a systematic review. Int J Mol Sci. 2022;23(7):3876.
- Omura-Matsuoka E, et al. Postischemic administration of angiotensin II type 1 receptor blocker reduces cerebral infarction size in hypertensive rats. Hypertens Res. 2009;32(7):548–553.
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145–153.
- Kjeldsen SE, et al. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J Clin Hypertens (Greenwich). 2007;7(3):152–158.
- Samuel S, et al. Reviving decades-old wisdom: longitudinal analysis of renin-angiotensin system inhibitors and its effects on acute ischemic stroke to improve outcomes. Am J Hypertens. 2024;37(7):531–539.
- Law MR, et al. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.
- Lim MA, et al. Immunosuppression for kidney transplantation: Where are we now and where are we going? Transplant Rev (Orlando). 2017;31(1):10–17.
- Novo G, et al. The role of the renin-angiotensin system in atrial fibrillation and the therapeutic effects of ACE-Is and ARBS. Br J Clin Pharmacol. 2008;66(3):345–351.
- Antoun I, et al. Hypertension and atrial fibrillation: bridging the gap between mechanisms, risk, and therapy. Medicina (Kaunas). 2025;61(2):362.
- Jia G, et al. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension. 2018;72(3):537–548.
- Leanca SA, et al. Left ventricular remodeling after myocardial infarction: from physiopathology to treatment. Life (Basel). 2022;12(8):1111. View this article via: CrossRef Google Scholar
- Kurdi M, Booz GW. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension. 2011;57(6):1034–1038.
- Lyu L, et al. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol. 2015;89(pt b):268–279.
- Verhaert DVM, et al. The bidirectional interaction between atrial fibrillation and heart failure: consequences for the management of both diseases. Europace. 2021;23(23 suppl 2):ii40–ii45.
- Amiel PJ, et al. Evaluating incident atrial fibrillation and incident heart failure as time-varying covariates for time-to-event analysis among adults 55 years and older in the multi-ethnic study of atherosclerosis (MESA) [published on February 3, 2025]. J Cardiac Fail. https://doi.org/10.1016/j.cardfail.2025.01.012.
- Healey JS, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45(11):1832–1839.
- Xu W, et al. Impact of renin-angiotensin-aldosterone-system inhibitor drugs on mortality in patients with atrial fibrillation and hypertension. BMC Cardiovasc Disord. 2022;22(1):141.
- Koniari I, et al. Atrial fibrillation in heart failure patients: An update on renin-angiotensin-aldosterone system pathway blockade as a therapeutic and prevention target. Cardiol J. 2023;30(2):312–326.
- Nehme A, Zibara K. Efficiency and specificity of RAAS inhibitors in cardiovascular diseases: how to achieve better end-organ protection? Hypertens Res. 2017;40(11):903–909.
- Patel VB, et al. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326.
- Sarkar S, et al. ACE 2/Ang (1-7)/Mas, non-conventional RAS axis: endogenous contributor of cardio, and reno-protective responses. J Cell Signal. 2024;5(3):149–161.
- Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717.
- Pitt B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321.
- Zannad F, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
- Pitt B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392.
- Sethi R, et al. Evidence for aldosterone antagonism in heart failure. Card Fail Rev. 2024;10:e15.
- Jhund PS, et al. Mineralocorticoid receptor antagonists in heart failure: an individual patient level meta-analysis. Lancet. 2024;404(10458):1119–1131.
- Musso CG, Jauregui JR. Renin-angiotensin-aldosterone system and the aging kidney. Expert Rev Endocrinol Metab. 2025;9(6):543–546.
- Laffer CL, et al. New insights into the renin-angiotensin system in chronic kidney disease. Circ Res. 2020;127(5):607–609.
- Kaltenecker CC, et al. Critical role of neprilysin in kidney angiotensin metabolism. Circ Res. 2020;127(5):593–606.
- Nakamura T, et al. Blocking angiotensin II ameliorates proteinuria and glomerular lesions in progressive mesangioproliferative glomerulonephritis. Kidney Int. 1999;55(3):877–889.
- Koo JW. Renal interstitial fibrosis and angiotensin inhibition. Electrolyte Blood Press. 2006;4(1):35–43.
- Bhandari S, et al. Renin–angiotensin system inhibition in advanced chronic kidney disease. New Eng J Med. 2022;387(22):2021–2032.
- Villain C, et al. Effectiveness and tolerance of renin-angiotensin system inhibitors with aging in chronic kidney disease. J Am Med Dir Assoc. 2022;23(6):998–1004.
- Herrera M, et al. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension. 2013;61(1):253–258.
- Miura S, et al. Recent progress in molecular mechanisms of angiotensin II type 1 and 2 receptors. Curr Pharm Des. 2013;19(17):2981–2987.
- Elased KM, et al. Brain angiotensin-converting enzymes: role of angiotensin-converting enzyme 2 in processing angiotensin II in mice. Exp Physiol. 2008;93(5):665–675.
- Bernstein KE, et al. Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline. J Clin Invest. 2014;124(3):1000–1012.
- Freund M, et al. Immunohistochemical localization of the angiotensin-(1-7) receptor Mas in the murine forebrain. Cell Tissue Res. 2012;348(1):29–35.
- Hassani B, et al. The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: foes versus allies. Cancer Cell Int. 2023;23(1):254.
- Lautner RQ, et al. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res. 2013;112(8):1104–1111.
- Nguyen G, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–1427.
- Triebel H, Castrop H. The renin angiotensin aldosterone system. Pflugers Arch. 2024;476(5):705–713.
- Faulkner JL, Belin de Chantemèle EJ. Mineralocorticoid receptor and endothelial dysfunction in hypertension. Curr Hypertens Rep. 2019;21(10):78.
- Feraco A, et al. Role of mineralocorticoid receptor and renin-angiotensin-aldosterone system in adipocyte dysfunction and obesity. J Steroid Biochem Mol Biol. 2013;137:99–106.
- Herbert KE, et al. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102(2):201–208.
- Schmid U, et al. Angiotensin II induces DNA damage in the kidney. Cancer Res. 2008;68(22):9239–9246.
- Kundura L, et al. Angiotensin II induces reactive oxygen species, DNA damage, and T-cell apoptosis in severe COVID-19. J Allergy Clin Immunol. 2022;150(3):594–603.
- Schupp N, et al. Angiotensin II-induced genomic damage in renal cells can be prevented by angiotensin II type 1 receptor blockage or radical scavenging. Am J Physiol Renal Physiol. 2007;292(5):1427–1434.
- Wang J, et al. The mTOR signaling pathway: key regulator and therapeutic target for heart disease. Biomedicines. 2025;13(2):397.
- Tabony AM, et al. Angiotensin II upregulates protein phosphatase 2Cα and inhibits AMP-activated protein kinase signaling and energy balance leading to skeletal muscle wasting. Hypertension. 2011;58(4):643–649.
- Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789–800.
- Yoshida T, et al. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J Biol Chem. 2013;288(33):23823–23832.
- Kim S, et al. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension. 2016;67(3):574–584.
- Tsubakimoto Y, et al. Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler Thromb Vasc Biol. 2009;29(10):1529–1536.
- Häfner S, et al. To live alone and to be depressed, an alarming combination for the renin-angiotensin-aldosterone-system (RAAS). Psychoneuroendocrinology. 2012;37(2):230–237.
- Terock J, et al. Living alone and activation of the renin-angiotensin-aldosterone-system: Differential effects depending on alexithymic personality features. J Psychosom Res. 2017;96:42–48.
- Carey Robert M. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38(6):1272–1277.
- Abadir PM, et al. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42(4):600–604.
- Düsing R. Pharmacological interventions into the renin-angiotensin system with ACE inhibitors and angiotensin II receptor antagonists: effects beyond blood pressure lowering. Ther Adv Cardiovasc Dis. 2016;10(3):151–161.
- Fournier A, et al. Cerebroprotection mediated by angiotensin II: a hypothesis supported by recent randomized clinical trials. J Am Coll Cardiol. 2004;43(8):1343–1347.
- Anderson C, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiova scular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10(1):43–53.
- Riccioni G. The role of direct renin inhibitors in the treatment of the hypertensive diabetic patient. Ther Adv Endocrinol Metab. 2013;4(5):139–145.
- Gorini S, et al. Role of aldosterone and mineralocorticoid receptor in cardiovascular aging. Front Endocrinol (Lausanne). 2019;10:584.
- Barrera-Chimal J, et al. Roles of mineralocorticoid receptors in cardiovascular and cardiorenal diseases. Annu Rev Physiol. 2022;84(1):585–610.
- Abadir P, et al. Unlocking the protective potential of the angiotensin type 2 receptor (AT2R) in acute lung injury and age-related pulmonary dysfunction. Biochem Pharmacol. 2024;220:115978.
- Ahmed HA, et al. Angiotensin receptor (AT2R) agonist C21 prevents cognitive decline after permanent stroke in aged animals-A randomized double- blind pre-clinical study. Behav Brain Res. 2019;359:560–569.
- Rathinasabapathy A, et al. The selective angiotensin II type 2 receptor agonist, compound 21, attenuates the progression of lung fibrosis and pulmonary hypertension in an experimental model of bleomycin-induced lung injury. Front Physiol. 2018;9:180.
- Shan B-S, et al. Attenuation of stroke damage by angiotensin II type 2 receptor stimulation via peroxisome proliferator-activated receptor-gamma activation. Hypertens Res. 2018;41(10):839–848.
- Royea J, et al. AT2R’s (angiotensin II type 2 receptor’s) role in cognitive and cerebrovascular deficits in a mouse model of alzheimer disease. Hypertension. 2020;75(6):1464–1474.
- Molaei A, et al. Mas receptor: a potential strategy in the management of ischemic cardiovascular diseases. Cell Cycle. 2023;22(13):1654–1674.
- Zoufaly A, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8(11):1154–1158.
- Laffin LJ, et al. Lorundrostat efficacy and safety in patients with uncontrolled hypertension. N Engl J Med. 2025;392(18):1813–1823.
- Morgan ES, et al. Antisense inhibition of angiotensinogen with IONIS-AGT-LRx: results of phase 1 and phase 2 studies. JACC Basic Transl Sci. 2021;6(6):485–496.
-
Version history
- Version 1 (November 3, 2025): Electronic publication



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)





