Advertisement
Review
Open Access |  10.1172/JCI191931
10.1172/JCI191931
Sex differences in the transition to chronic pain
Angela F. Smith,1 Ashley N. Plumb,2 Giovanni Berardi,1 and Kathleen A. Sluka1
1Department of Physical Therapy and Rehabilitation Science, University of Iowa, Iowa City, Iowa, USA.
2Department of Neuroscience, University of Texas at Dallas, Richardson, Texas, USA.
Address correspondence to: Kathleen A. Sluka, Roy J Carver Chair in Neuroscience, 500 Newton Road, 1-252 Medical Education Building, Iowa City, Iowa 52242-1089, USA. Email: kathleen-sluka@uiowa.edu.
Find articles by Smith, A. in: PubMed | Google Scholar
1Department of Physical Therapy and Rehabilitation Science, University of Iowa, Iowa City, Iowa, USA.
2Department of Neuroscience, University of Texas at Dallas, Richardson, Texas, USA.
Address correspondence to: Kathleen A. Sluka, Roy J Carver Chair in Neuroscience, 500 Newton Road, 1-252 Medical Education Building, Iowa City, Iowa 52242-1089, USA. Email: kathleen-sluka@uiowa.edu.
Find articles by Plumb, A. in: PubMed | Google Scholar
1Department of Physical Therapy and Rehabilitation Science, University of Iowa, Iowa City, Iowa, USA.
2Department of Neuroscience, University of Texas at Dallas, Richardson, Texas, USA.
Address correspondence to: Kathleen A. Sluka, Roy J Carver Chair in Neuroscience, 500 Newton Road, 1-252 Medical Education Building, Iowa City, Iowa 52242-1089, USA. Email: kathleen-sluka@uiowa.edu.
Find articles by Berardi, G. in: PubMed | Google Scholar
1Department of Physical Therapy and Rehabilitation Science, University of Iowa, Iowa City, Iowa, USA.
2Department of Neuroscience, University of Texas at Dallas, Richardson, Texas, USA.
Address correspondence to: Kathleen A. Sluka, Roy J Carver Chair in Neuroscience, 500 Newton Road, 1-252 Medical Education Building, Iowa City, Iowa 52242-1089, USA. Email: kathleen-sluka@uiowa.edu.
Find articles by Sluka, K. in: PubMed | Google Scholar
Published June 2, 2025 - More info
J Clin Invest. 2025;135(11):e191931. https://doi.org/10.1172/JCI191931.
© 2025 Smith et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Chronic pain affects more than 50 million Americans, with women disproportionately affected by severe pain, pain interference, and overall disability. The development of chronic pain is multifactorial and often begins with an incident of acute pain associated with an injury or a surgical procedure that transitions to persistent pain lasting for months or years. Despite this, there are limited clinical studies investigating sex differences in predictors and biomarkers for the transition to chronic pain. Several preclinical animal models have been developed to gain a better understanding of the mechanisms for the transition to chronic pain, and several sex-specific mechanisms have been identified across multiple systems. These preclinical models generally involve a multiple-insult approach, in which a priming insult enhances sensitivity to a subsequent induction stimulus. There is emerging evidence from preclinical research for several male-specific and female-specific mechanisms, as well as several studies showing shared mechanisms. Here, we review the clinical and preclinical literature covering sex differences in the periphery and immune system, the central nervous system, and the endocrine system related to the transition to chronic pain. We further highlight gaps in the literature and provide recommendations for future research to understand sex-specific differences in the transition to chronic pain.
-
Introduction
Chronic pain affects more than 50 million Americans and is a leading cause of disability, with associated health care and lost-productivity costs totaling $600 billion in the U.S. annually (1). It is associated with reduced productivity and quality of life, which impacts the individual, their family, and society (2). The development of chronic pain is multifactorial; for some individuals there is an absence of clear etiology, while for others it begins with an incident of acute pain associated with an injury or a surgical procedure that transitions to pain and persists for longer than 3 months (3–6). While there are clear sex differences in the prevalence of chronic pain, and accumulating evidence suggests that pain mechanisms across multiple physiological systems are dependent on biological sex, few studies have examined sex differences in predictors or treatments for chronic pain.
A number of prior reviews focused on clinical differences between sexes, suggesting unique sex-specific mechanisms of chronic pain (7–11). However, these have not specifically focused on the acute-to-chronic–pain transition. Therefore, the purpose of this review is to highlight the current understanding of sex differences in the transition to chronic pain in clinical and animal studies, and provide recommendations for future research in the field. We will focus on predictors and risk factors identified in human subjects and underlying mechanisms from animal models of the transition to chronic pain. We use the terms ‘men’ and ‘women’ to describe biological sex for human subjects, which are collected through self report in the available literature. For animal studies, we use the terms ‘male’ and ‘female’ to define biological sex.
-
Sex differences in pain
Over 50% of chronic pain conditions are more prevalent in women, whereas approximately 20% of these conditions are more prevalent in men (12, 13). In addition, women are more sensitive to experimental pain stimuli (14), and those with chronic pain often experience more severe pain, pain interference, and widespread pain (15, 16). Overall, women present with greater disability and loss of function due to pain compared with men (15, 17, 18).
It is well known that sex hormones can influence pain in both sexes (19, 20). In both men and women, sex hormone levels peak in the 20–40 year age range. In men, there is a gradual decline in sex hormones with age, whereas women experience an abrupt decrease after menopause. Although the incidence of chronic pain is similar between both sexes prior to puberty (21), the incidence increases in women after puberty and varies through menopause (19, 22). In addition, both clinical and preclinical studies suggest that the prominent sex hormone in males, testosterone, is protective against pain, while mixed effects are noted with the female sex hormone, estrogen (23–27). In conditions such as fibromyalgia, rheumatoid arthritis, and osteoarthritis, lower levels of testosterone are correlated with poorer health status and greater pain severity (28–31). On the other hand, estradiol, the primary form of estrogen in the body during reproductive years, has mixed effects on pain, and these effects may depend on the pain condition (19, 32). Interestingly, perimenopausal women often report greater pain, while postmenopausal women report either increases or decreases in their pain, depending on the condition and intensity of pain (33).
Importantly, there are sex differences in the delivery and effectiveness of treatments for chronic pain (13, 34–42). A secondary analysis of migraine clinical trials using gepants, small molecule calcitonin gene related peptide (CGRP) inhibitors, showed that women experience greater reductions in pain compared with men, and gepants demonstrated effectiveness for acute migraine in women only (43). Sex differences in response to opioids for pain relief have been reported in the literature, suggesting μ-opioid agonists are less potent in women and that women consume less opioids postoperatively; however, these findings are not consistent (7, 34, 44). Metaanalyses show no differences in response to opioids for acute pain, yet this may depend on the opioid used and type of pain treated. Consistently, women used fewer opioids than men for acute pain, and women received lower doses of opioids for chronic pain, particularly those over the age of 45 with the same level of pain (35). It is unclear if these differences for chronic pain are due to a better response to opioids among women or to providers prescribing lower doses to women. For nonpharmacological and interdisciplinary treatments there are reported sex differences, yet these are not consistent among studies, with some showing longer term and greater reduction in men and others showing greater effects in women (37–40, 42). Overall, the majority of clinical studies have not disaggregated or analyzed data by sex, but rather have controlled for sex in the analysis, making it difficult to fully interpret sex differences in response to treatment. Secondary analysis of existing datasets, like that done for gepants, may yield useful data and more definitive results.
-
Acute-to-chronic–pain transition incidence and predictors
Across the human lifespan, 20%–70% of individuals will develop chronic pain that persists beyond the usual recovery time from an acute injury, surgical procedure, or illness (45–47). Estimates vary depending on the type of acute injury and the methods used to evaluate pain (48). For example, 6 months after knee replacement surgery for osteoarthritis, 16% of individuals reported pain at rest, while 32% reported pain with movement or activity (49). Similarly, the incidence of chronic postthoracotomy pain at 3 and 6 months ranged from 20%–80%, due to varying methods of pain assessment, as only 2 out of 31 studies used the same approach to measure pain severity (50). Thus, in addition to high-quality clinical trials, use of standardized pain assessments across trials will lead to improved understanding of the acute-to-chronic–pain transition (51).
The underlying mechanisms of the transition to chronic pain are likely multifactorial, involving biological and psychosocial factors, and have been reviewed elsewhere (52) (Figure 1). The transition to chronic pain is strongly predicted by higher levels of pain during the acute phase, the presence of widespread pain, or higher movement-evoked pain (49, 52, 53). However, it is unclear if certain individuals are predisposed to developing chronic pain after an acute injury or if factors associated with acute injury predispose an individual to the development of chronic pain. Understanding the factors that make an individual susceptible or resilient to the development of chronic pain will guide development and implementation of treatments to reduce the risk of acute-to-chronic–pain transition.
 Figure 1
Figure 1Biopsychosocial factors that could promote the transition to chronic pain in humans. Biopsychosocial factors implicated across the spectrum of acute-to-chronic–pain transition in humans. After an injury during the acute phase and the subacute healing phase, there may be factors that predict who will transition to a chronic pain phase, referred to as predictive and prognostic biomarkers. A number of biological and self-report measures likely contribute to the transition to chronic pain. Sex is routinely a risk factor for the transition to chronic pain and pain severity. However, while a number of studies have investigated factors that promote the transition to chronic pain, few studies have investigated differences between the sexes. There are likely sex differences across biological and psychosocial factors that contribute to the transition to chronic pain, and future studies should examine sex-specific risk and resilience to development of chronic pain.
There is strong evidence that a number of psychosocial factors predict the transition to chronic pain in a variety of pain conditions, including stress, depression, anxiety, pain catastrophizing, early-life experiences, substance use disorders, and trauma (52, 54, 55). Although the evidence is less substantial than that related to negative psychosocial factors, several studies identified positive psychosocial factors that may reduce the risk for transition to chronic pain, including positive affect, resilience, adaptive coping, engagement with valued activities, and increased physical activity (56–58). Notably, traits associated with positive affect can attenuate biological and/or psychosocial risk factors such as widespread pain, depression, and fear of movement in favor of reduced pain and improved function (59–61).
In contrast to the literature investigating the influence of pain and psychosocial factors on the transition from acute to chronic pain, there has been limited investigation into biological factors or biomarkers. Animal research has driven much of our knowledge; however, there has been an increased focus on conducting mechanistic studies in humans. For example, baseline neuroimaging measures such as white matter fractional anisotropy, gray matter volume of the medial prefrontal cortex, and functional connectivity of the cortico-striatal and hippocampal areas predicted the transition from acute to chronic pain 1 year after an acute back pain episode (62–67). The clinical literature suggests that some genetic, protein, lipid, metabolite, and nervous system factors may relate to the pain experience; however, most of these studies have investigated these markers in isolation and in smaller sample sizes (68–74).
The development of chronic pain is likely multifactorial, resulting from an interaction between biological, psychological, and social factors. Commonly, many risk (priming) factors are present and involve both biological and psychosocial mechanisms. For example, individuals with substance use disorder have increased risk for the transition to chronic pain due to alterations in neurobiological processes, social functioning, and cognitive experiences (54, 75, 76). Similarly, stress induces psychological and physiological changes, perhaps contributing to the uncertainty of cortisol regulation as a biomarker of the development of chronic pain (77, 78) following traumatic injury. Thus, exploration of the numerous biomarkers and factors that contribute to the transition to chronic pain presents significant challenges, and active research in this area is currently underway (52).
-
Sex-specific differences in the transition to chronic pain
Despite a large body of literature identifying factors associated with the acute-to-chronic–pain transition, whether sex differences exist among these factors has yet to be elucidated. While over 90% of human studies include both men and women, the majority of clinical pain research does not analyze or disaggregate data by sex (34, 79–86) (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI191931DS1). Female sex is routinely identified as a predictor for development of chronic pain in adolescents (87–89) but not children (90), suggesting that sex hormones present after puberty play a role in transition to chronic pain (34, 81–86, 91). Further, more women than men developed chronic pain after an emergency department visit for acute pain (15.5% versus 8.7%, respectively) or after thoracic surgery (53.1% versus 38.0%, respectively) (92, 93). Early life experiences involving trauma and stress may have a more pronounced impact on the risk of developing visceral pain with irritable bowel syndrome in women compared with men (94). Thus, women may be more vulnerable to the transition to chronic pain after an acute stressor or injury, which may depend on sex hormones.
Investigations into if psychosocial predictors for the transition to chronic pain differ by sex have been extremely limited. One study found that women have higher levels of pain catastrophizing (characterized by pain magnification, rumination on pain, and pain-related helplessness) compared with men. When reporting pain at a level greater than 1 (on a 0–10 scale), pain catastrophizing predicted development of chronic pain in both men and women; however, when pain was defined as at least 4, pain catastrophizing predicted development of chronic pain only for women (92). Additionally, preoperative chronic pain significantly contributed to postoperative pain in women but not men (92). Whether there are sex differences in other psychosocial predictors remains to be determined.
Thus, emerging evidence suggests that there may be differences in psychosocial predictors of the transition to pain, however, few studies have analyzed clinical data sets for sex, and no studies examined sex differences in biomarkers as a primary aim. Understanding unique predictors of chronic pain between the sexes and the underlying biological and psychosocial mechanisms of chronic pain are critical for tailoring pain management strategies and improving outcomes for both men and women suffering from pain-related disorders across their lifespan. To gain a better understanding of potential sex differences within biological contributors to the transition to chronic pain, we will next discuss the use of animal models that focus on the transition to chronic pain.
-
Animal models of the transition to chronic pain
Animal models of the transition to chronic pain generally involve multiple insults, in which a prior insult or stressor enhances pain sensitivity to a subsequent stimulus, referred to as a priming stimulus and an induction stimulus, respectively. The stimuli are designed to mimic predictors and insults from clinical conditions, including inflammatory stimuli, surgical pain, movement pain, chronic opioid use, early-life insult, or stress (53, 95–103). The priming stimuli create a vulnerability to develop elevated pain behaviors in response to the induction stimuli. The induction stimuli are given after initial hyperalgesia from the priming stimuli resolve and often produce no or little response on their own in unprimed animals but consistently produce a long-lasting, out-of-proportion response in primed animals, thus modeling a chronic pain condition. These models therefore allow for examination of mechanisms underlying the transition to chronic pain, including risk, induction, and resilience factors, as well as exploration of potential novel therapeutics (Supplemental Table 2). Many multiple-insult animal models were initially established and characterized in males and have not been evaluated for behavioral or mechanistic sex differences. However, a few models that demonstrate sex differences in behavior or mechanisms have broadened our understanding of sex differences in the transition to chronic pain (Figure 2).
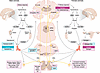 Figure 2
Figure 2Sex-specific pathways, systems, and mechanisms identified in the transition to chronic pain. Their interaction likely results in a multifactorial process that promotes the transition to chronic pain. Endocrine system release of hormones, including sex hormones, prolactin, and cortisol, into the blood stream can promote this transition via interaction with the nervous and immune systems. In males, testes release testosterone systemically in response to hormones released from the hypothalamus and the anterior pituitary, producing a protective effect through activation of ARs to reduce pain. In females, ovaries release estrogen in response to hypothalamic and anterior pituitary hormonal signals, producing mixed effects that depend on the model. At the site of insult, HPA axis–derived hormones activate intracellular messengers, macrophages, and receptors that can promote the transition to chronic pain, with different mechanisms observed in male versus female animals. Similarly, in the spinal cord, intracellular messengers mediate the transition to chronic pain in a sex-dependent manner, while involvement of microglia and dopamine DRD5 receptors is specific to male mice. In females, tonic KOR activation in the spinal cord protects from development of chronic pain. Unique mechanisms are found supraspinally where, in males, aromatization of testosterone to estradiol activates ER-α, which protects against development of pain, while, in females, increases in SERT promote the transition to chronic pain. PKC in the amygdala plays a unique role in males. While few mechanisms have been identified in humans, roles of testosterone and estrogen generally mirror those found in animals, and pain catastrophizing is more likely to promote the transition in women. Pink boxes indicate risk/transition factors and blue boxes indicate protective factors. Not shown here: mechanisms found in both male and female mice (see Figure 3). GnRH, gonadotropin releasing hormone; CRH, corticotropin releasing hormone; LH, luteinizing hormone; FSH, follicular stimulating hormone; ACTH, adrencorticootropic hormone; GCR, glucocorticoid receptor; STT, spinothalamic tract; DLPT, dorsolateral pontine tegmentum; PAG, periaqueductal gray; CC, cingulate cortex; S1/2, somatosensory cortex 1 and 2.
To mimic acute injury, inflammatory hyperalgesic priming models used carrageenan or CFA as a priming stimulus, which produced hyperalgesia in males, but not females, upon subsequent foot shock (104) or prostaglandin E2 (PGE2) (105). Subsequent studies showed the inflammatory cytokine IL-6 or paw incision as the priming stimulus, followed by PGE2 as the induction stimulus, produced hyperalgesic priming equally in both sexes (106–108). Alternatively, two injections of acidic saline (pH 4.0) into the muscle given 2–5 days apart, but not 10 days apart, produced noninflammatory hyperalgesic priming in both sexes (95, 109–111). As a model of activity-induced hyperalgesia, two intramuscular injections of acidic saline (pH 5.0) five days apart were combined with fatiguing muscle contractions prior to the second injection, none of which produced hyperalgesia alone (96). This model produced sex-specific hyperalgesic priming wherein females developed a more robust, widespread, and longer-lasting hyperalgesia than males. Additionally, females were more susceptible to hyperalgesia in this model, with spatially and temporally remote stimuli capable of inducing hyperalgesia (96). A repetitive ischemia reperfusion injury model used two ischemic insults seven days apart, where the second insult produced longer lasting hyperalgesia than the first. This similarly caused a longer-lasting muscle hyperalgesia in females than males (112). Thus, multiple peripheral insults within a critical window after resolution of initial hyperalgesia from the priming stimulus can produce long-lasting hyperalgesia, some of which present with sex-specific behavioral profiles.
To mimic stress-related priming, repeated stress followed by injection of a nitric oxide (NO donor) in adult mice led to development of migraine-like symptoms in both sexes (102, 113). Repeated stress also induced muscle and visceral hyperalgesia in males, although this was untested in females (114, 115). Early life stress or injury as a priming stimulus can serve as a biologically relevant model of the transition to chronic pain, as infants who experience painful procedures may have increased pain in adulthood (116–120). In animal models, an early life stressor can produce an exaggerated pain response upon reinjury in adulthood (116, 117, 120). Multiple variations of neonatal priming exist, including stress, chemotherapy exposure, inflammation, paw incision, and needle stick, many of which demonstrate a sex difference (103, 121–125). In adolescent mice, chronic alcohol exposure followed by withdrawal produced hypersensitivity in both sexes that is longer lasting than that in adult mice experiencing withdrawal (126). While the extent of priming differs depending on the stimuli used, younger animals are generally more susceptible to priming (53, 116, 117), suggesting that increased plasticity associated with neurodevelopment provides a sensitive window during which mechanisms involved in the transition to chronic pain more readily occur.
Changes in endogenous inhibition in the spinal cord may underly the transition to chronic pain. Latent sensitization models involve a painful insult as a priming stimulus, with blockade of endogenous inhibition, typically opioid receptors, as an induction stimulus after resolution of initial hyperalgesia (127, 128). In this model, spinal or systemic inhibition of μ-opioid receptors (MOR), systemic inhibition of δ-opioid receptors (DOR), or systemic inhibition of μ-δ heteromers reinstated hyperalgesia equally in both sexes. However, spinal inhibition of κ-opioid receptors (KOR) reinstated hyperalgesia more robustly and at lower doses in females than in males (129, 130). Thus, loss of endogenous pain inhibition is one mechanism for the transition to chronic pain with both shared and sex-specific mechanisms.
A growing body of evidence suggests that multiple priming stimuli can enhance sensitivity to a subsequent induction stimulus to result in long-term hyperalgesia and model the transition to chronic pain, suggesting that the transition to chronic pain is multifactorial. Using these models, sex-specific mechanisms have been shown in the periphery and immune system, the central nervous system, and the endocrine system (Figure 2); however, it should be pointed out that there are also shared mechanisms between the sexes (Figure 3). Understanding mechanisms that contribute to the transition to chronic pain in both sexes is critical to development of strategies to prevent chronic pain conditions. Thus, this review focuses on studies that examine mechanisms during the priming and induction of chronic pain, rather than those that reverse the hyperalgesia once developed.
 Figure 3
Figure 3Summary of identified mechanisms involved in promoting and protecting from the transition to chronic pain. Mechanisms identified in animals in the transition to chronic pain from the peripheral and central nervous systems, immune system, and endocrine system. Animal studies have provided evidence of a variety of underlying mechanisms involved in the transition to chronic pain (transition factors) or prevention of chronic pain (resilience factors). Importantly, a number of studies have examined both males and females and identified some sex-specific pathways across all systems. Those with known sex-specific mechanisms are labeled with an “(m)” to show this only occurs in males or an “(f)” to show this mechanism occurs in females. <m and <f indicate that the associated factor contributes to a greater degree in males or females, respectively. It should be noted however, that there are a number of mechanisms that are found in both sexes, and that the sex-specific differences noted may be dependent on the animal model or species used. An overview of the animal literature including mechanism and model details can be seen in Supplemental Table 2. Mechanisms involved in the transition to chronic pain involve peripheral, central, immune and endocrine factors.
-
Peripheral neural and immune mechanisms in animal models
It has become increasingly clear that peripheral neural and immune mechanisms are involved in the transition to chronic pain (131, 132). Intracellular messengers can modify receptor function and initiate gene transcription and protein translation to produce long-lasting changes in neuronal excitability. At the level of the nociceptor, there is substantial evidence for the intracellular messenger PKC-ε in the generation of hyperalgesic priming. Priming with a PKC-ε activator produced an exaggerated pain response to PGE2 injection in males but not females (105). However, priming with activators of intracellular messengers downstream of PKC-ε — IP3, CAMKII, and ryanodine receptors — produced hyperalgesic priming to PGE2 in both sexes, although at lower doses in females (133–136). Inhibition of the intracellular messengers — PKC-ε, MEK, ERK, and CAMKII — during induction prevented hyperalgesic priming in both sexes (136). In a hyperalgesic priming model that uses chronic opioid agonism as the priming stimulus, coinhibition of MAPK and Src reversed priming in males but not females (99). In parallel, there was increased activation of ERK (measured as pERK) in sensory neurons in a noninflammatory hyperalgesic priming model to a greater extent in males than in females (137). AU-rich element RNA-binding protein, which regulates translation, was more highly phosphorylated in females than males in a repeated ischemia reperfusion injury model; inhibition in females attenuated hyperalgesia, while overexpression in males potentiated hyperalgesia (112). In contrast, local (paw) inhibition of translation reduced ryanodine hyperalgesic priming in both sexes (136). Thus, in the periphery, priming may produce changes in intracellular messengers that modulate production of proteins to make nociceptors more sensitive to a subsequent stimulus and result in hyperalgesia, with some sex-specific mechanisms.
Brain-derived neurotrophic factor (BDNF) plays a significant role in hyperalgesic priming in a sex-specific manner. Peripheral blockade of BDNF during induction in the IL-6 or activity-induced hyperalgesic priming model prevented hyperalgesia in males but not females (138, 139). However, in activity-induced hyperalgesic priming, BDNF was upregulated in the DRG of both males and females, suggesting that BDNF may play a role in females, even if blockade is not sufficient to prevent hyperalgesia (138). Surprisingly, in IL-6 hyperalgesic priming, this sex difference for BDNF was only present in mice; blockade of BDNF prevented IL-6 hyperalgesic priming in both sexes in rats (139). Together these data support that BDNF contributes to hyperalgesic priming in males, and may play some role in females, although the data is mixed.
In activity-induced hyperalgesic priming, males and females present with both shared and unique immune mechanisms in the development of hyperalgesia. In both sexes, depletion of macrophages in muscle prevented the development of hyperalgesia, showing a role for local macrophages in induction of priming (96, 140, 141). However, blockade of the immune purinergic receptor P2X7 and its downstream pathway (NLRP3/caspase-1) in muscle during induction reduced hyperalgesia in males but not females (140). Despite this behavioral sex difference, there was an equivalent upregulation of genes in the P2X7 pathway in both sexes, suggesting alternative mechanisms or a suppression pathway in females (140). On the other hand, in females, but not in males, there was an upregulation of MHC II, a cell-surface signaling molecule expressed in macrophages, and blockade of MHC II during induction prevented activity-induced pain (142). Not all pathways present sex differences, as blockade of IL-1β, P2X4, and ASIC3 in muscle prevents activity-induced hyperalgesic priming in both sexes (140, 141, 143). Similarly, in a model of neonatal incision priming, macrophage-deficient mice of both sexes did not develop hyperalgesia to a second incision in adulthood (123). Further, adult mice that received neonatal incision had altered macrophage epigenetic and mRNA signatures, including for nerve growth factor receptor (NGFR), and macrophage-specific NGFR knockout attenuated the hyperalgesic priming to a second incision given in adult mice (123). These studies highlight how alterations in the immune system, particularly macrophages, can influence the transition to chronic pain with both sex-specific and shared mechanisms.
While scientists generally examine peripheral mechanisms that promote a transition to chronic pain, it should be noted that there may also be factors that promote recovery. Indeed, we have shown that regular exercise prevents the transition to chronic pain in both male and female mice through multiple mechanisms (144–148). While sex-specific factors clearly can modulate the transition to chronic pain, there are likely sex-specific resilience factors yet to be discovered.
-
Central neural and immune mechanisms in animal models
Sites within the central nervous system are involved in nociception and pain, some of which have been investigated for sex differences in the transition to chronic pain (149) (Figure 2). The spinal cord has been extensively studied as it receives nociceptive input from injured sites and integrates pain modulation from supraspinal sites. Additionally, the amygdala has emerged as a key area in the emotional aspects of pain, and recent work shows its involvement in the transition to chronic pain. Lastly, the thalamus, which relays nociceptive information from the spinal cord to the cortex, has also been investigated for its role in the transition to chronic pain. Within each of these sites, identified sex-specific mechanisms in the transition to chronic pain are discussed below.
Spinal cord. Intracellular signaling in the spinal cord plays a role in the transition to chronic pain by enhancing long-term changes in neuronal excitability through modification of receptors and increases in gene transcription. In noninflammatory hyperalgesic priming, knockout or inhibition of protein kinase M-ζ (PKM-ζ) in the spinal cord prevented chronic pain in male but not female mice (150). Targeting protein kinase A (PKA) in the same noninflammatory priming model also prevents development of pain in males but not females (151, 152). Similarly, in the latent sensitization model, reinstatement of hyperalgesia after blockade of KOR was prevented by spinal inhibition of PKA, and PKA activation alone reinstated hyperalgesia in male but not female mice (153). As in the periphery, in female mice, blockade of protein translation prevented IL-6 hyperalgesic priming in males but not females (106). Taken together, these studies suggest that, within the spinal cord, intracellular mechanisms are differentially involved in pain in males and females at different time points in the transition to chronic pain.
Spinal dopamine both inhibits and facilitates pain (154). In IL-6 hyperalgesic priming, spinal blockade of both dopamine D1 and D5 receptors delayed the transition to chronic pain in both sexes, while a D5 receptor knockout prevented development of hyperalgesic priming only in males (107, 155). This suggests that dopamine D1/D5 receptors differentially contribute to the development of chronic pain in males and females. In contrast, in neonatal incision priming, dopamine D1/D5 receptors contributed to increased long-term potentiation (a measure of central activity) in the spinal cord (156, 157). In D5-knockout mice, facilitation of long-term potentiation was equally reduced in both sexes (157). These data suggest that spinal dopamine plays a role in the transition to chronic pain by reducing central excitability in both sexes, and D1 and D5 receptors may have a sexually dimorphic role.
Within the spinal cord, microglia activation in male mice plays a role in hyperalgesia in a variety of animal models of pain (158–161). In animals with neonatal incision priming, inhibiting microglia at the time of neonatal incision prevented hyperalgesic priming to adult reincision and reduced expression of genes associated with microglia proliferation in the spinal cord in adult males but not females (103, 124). Similarly, spinal blockade of the microglial purinergic receptor P2X4 prevented IL-6 hyperalgesic priming in only males (162). Thus, spinal microglia appear to play a more consequential role in the transition to chronic pain in males.
Supraspinal. Compared with research in the spinal cord, less work has investigated potential supraspinal mechanisms underlying the transition to chronic pain, particularly for sex differences. Like in the peripheral nervous system and spinal cord, intracellular messengers in the amygdala have been implicated in the transition to chronic pain. In an incision hyperalgesic priming model, there was increased expression of PKC-ζ in the basolateral amygdala, while inhibiting atypical PKC or genetic deletion of PKC-ζ prevented hyperalgesia to PGE2 after plantar incision in male, but not in female, mice (108). In the latent sensitization model, systemic naltrexone increased Fos expression in the central nucleus of the amygdala similarly in both sexes, a portion of which express PKC-δ, showing increased activation of the amygdala in primed animals (163). Thus, PKC isoforms within the amygdala appear to play a role in the transition to chronic pain, similar to that observed in sensory neurons and the spinal cord, some of which are sex dependent.
As noted for the periphery and spinal cord, not all pathways show sexual dimorphism. In the amygdala, inhibition of GluA2 prevented hyperalgesic priming following paw incision in both sexes (108), chemogenic inactivation of somatostatin-expressing neurons during priming prevented development of noninflammatory hyperalgesic priming (164), and microinjection of a competitive MOR agonist into the CeA reinstated hyperalgesia in both sexes (163). In the paraventricular thalamus, Cav3.2-dependent activation of ERK during the priming phase, but not during the maintenance phase, was necessary for initiation of the noninflammatory hyperalgesic priming model in both sexes (165). Thus, emerging reports suggest that the amygdala and the paraventricular thalamus are involved in the transition to chronic pain, with some pathways and receptors showing sexual dimorphism that varies depending on brain region, pathway, and model.
-
Endocrine mechanisms in animals
The endocrine system is increasingly being recognized for its role in pain. The endocrine system is made up of multiple glands that release hormones that can produce effects throughout the body (166, 167) (Figure 2). Researchers studying preclinical sex differences in pain have primarily focused on sex steroids produced by the gonads, in particular, circulating testosterone in males and estrogen in females and the hypothalamic-pituitary-adrenal (HPA) axis.
-
Sex hormones
Testosterone. The male sex hormone, testosterone, has routinely been shown be protective in pain models, including hyperalgesic priming models. In males, removal of endogenous testosterone with a gonadectomy prolonged hyperalgesic priming in some models (IL-6 and activity-induced hyperalgesic priming) (24, 106), but had no effect in the inflammatory hyperalgesic priming model (105). On the other hand, testosterone administration to females attenuates hyperalgesic priming in the activity-induced pain model (24). In both males and females, exercise protects against development of activity-induced hyperalgesic priming through androgen receptor–mediated mechanisms (145). This suggests that testosterone protects against the development of pain in both sexes, and sex differences in the transition to chronic pain may be due, in part, to different levels of circulating testosterone between the sexes.
One region shown to be involved in this protection is the rostral ventromedial medulla (RVM). In the RVM of males, testosterone is aromatized into estradiol to activate estrogen receptor α (ER-α) and protect against the transition to chronic pain. However, blockade of ER-α in the RVM in females had no effect on activity-induced hyperalgesic priming (168). In females, exogenous testosterone reduced the increases in the serotonin reuptake transporter (SERT) normally observed in the RVM in the activity-induced priming model (169). Thus, unique pathways within the RVM mediate the protective effects of testosterone on hyperalgesic priming in males and females.
Estrogen. The female sex hormone estradiol has been extensively studied in hyperalgesic priming and shows mixed results. In animals with carrageenan or PKC-ε hyperalgesic priming, administration of estradiol suppressed hyperalgesia in males, while ovariectomy or ER-α inhibition facilitated hyperalgesic priming to PGE2 in females (105, 135). In contrast, ovariectomy attenuated IL-6 or noninflammatory hyperalgesic priming (106, 110) but had no effect in the activity-induced priming model (96). These data suggest that estrogen may be protective in males, yet its effects are highly model dependent in females.
A few studies have examined if the mechanisms underlying the transition to chronic pain depend on estrogen or activation of estrogen receptors. Females were more sensitive to priming by the intracellular messengers ryanodine or IP3, an effect regulated by ER-α (133). Further, local blockade of translation prevented hyperalgesic priming in females that was abolished by ovariectomy, but had no effect in males (106). Thus, estrogen may modulate some of the female-specific mechanisms observed in hyperaglesic priming models.
-
HPA axis
The HPA axis is a key part of the body’s stress response, releases cortisol, and has been implicated in chronic pain (170). Interestingly, in animals primed with the chemotherapy drug oxaliplatin, PGE2-induced hyperalgesia was reduced by adrenalectomy; however, only male rats were tested (171). In this hyperalgesic priming model, reduction of β2-adrenergic receptors or glucocorticoid receptors prevented the hyperalgesia, suggesting a role for the HPA axis (171). Similarly, in a repeated stress migraine model (combining repeated stress with NO donor injection), blockade of corticosteroid production or glucocorticoid receptors prevented hyperalgesia in both sexes, while repeated corticosterone injections reproduced migraine behaviors only in female mice (113). These data show a role for the HPA axis in the transition to chronic pain, yet whether there are sex-specific mechanisms are yet to be determined.
Prolactin, a neuroendocrine hormone secreted from the pituitary that can modulate the HPA axis, is involved in hyperalgesic priming (106). Specifically in females, but not in males, priming with prolactin produced prolonged hypersensitivity to PGE2, while prolactin receptor knockout in sensory neurons prevented IL-6 hyperalgesic priming. Interestingly, chemical removal of the pituitary had no effect on hyperalgesia following induction with PGE2 in females and slightly prolonged the response in males (106). These data suggest that local synthesis of prolactin and its receptor play a critical role in sensory neurons in the transition to chronic pain.
As is the case in the peripheral nervous and immune systems, efforts often focus on mechanisms that contribute to the transition to chronic pain. However, neonatal handling produces resilience to oxaliplantin and stress priming in males, which is dependent on testosterone and adrenal gland signalling (171, 172). Thus, the endocrine system, particularly testosterone and activation of the HPA axis, play a critical role in the resilience to development of chronic pain, and future studies in this area may identify shared and sex-specific resilience mechanisms.
-
Outlook on sex-dependent factors in chronic pain
As highlighted in this review, there is limited understanding of the mechanisms and factors leading to the transition from acute to chronic pain, particularly in humans. Emerging evidence shows that some predictors and mechanisms may be sex specific and that sex-specific mechanisms may depend on the pain condition (Figure 3). Indeed, the periphery and immune system, the central nervous system, and the endocrine system all play a role in the sexual dimorphism in the transition to chronic pain and likely act together to promote the transition to chronic pain (Figure 2). Understanding these sex-specific mechanisms could lead to improved pain management using a more individualized approach. Below, we outline a number of potential pathways to improve our understanding of sex-specific mechanisms not only in the transition to chronic pain, but for the scientific community as a whole.
First, sex as a biological variable needs to be routinely considered in both preclinical and clinical studies investigating the transition to chronic pain; specifically, sex needs to be factored into research design, analysis, and reporting in cell culture, animal, and human studies. While funding agencies across the globe (e.g., the U.S., Europe, Canada, and Australia) mandated nearly a decade ago that all research grants consider sex as a biological variable, the most recent literature reviews in the journal PAIN show that only about 50% of animal studies include males and females, and less than 20% of clinical studies report results by sex (169). Beyond the requirements of funding agencies, it will take a cultural change in scientific research to fully implement this approach. Reviewers of grants and manuscripts need to be aware of the increased workload that is required for basic science and clinical studies that are powered to examine sex-specific differences and scale their expectations appropriately. Funding agencies need to enforce the inclusion of and support the reporting of sex-specific differences. Importantly, we believe that the biggest impact to promote change will come from scientific journals. Journals should require the use of sex as a biological factor in both basic science and clinical studies, and, unless adequately justified, should encourage the reporting of effects by sex or sharing of all sex-specific data.
Second, understanding sex as a biological variable in both behavioral output and mechanisms is critical to fully understand the transition to chronic pain and to ultimately provide individualized patient-centered care. Given the variability of underlying biological and psychosocial mechanisms, it will be increasingly important to power studies to detect sex-specific effects. This means that the scientific community will need to recalibrate its expectations about what should be included in an experimental design. For example, we need to understand how sex differences affect changes in pain across the lifespan and uncover sex-specific biopsychosocial mechanisms to develop tailored treatments for males and females with chronic pain. These differences may affect observed sex biases across pain management and science (79, 173, 174).
Last, in chronic pain research, the use of large datasets, like the Acute to Chronic Pain Signatures (A2CPS), the UK Biobank, or the National Health and Nutrition Examination Survey (NHANES), are ideal for performing high-powered, sex-specific analyses; any study using these data should routinely perform an analysis by biological sex. As an example, the A2CPS is a unique dataset that focuses on the transition from acute to chronic pain in men and women following surgery (175). The A2CPS research initiative collects biomarkers before and after surgery and examines them for factors that predict the transition to chronic pain 6 months later. Biomarkers and predictors are collected across multiple domains, including pain and associated symptoms; psychosocial measures; brain imaging; quantitative sensory testing; and a variety of omics platforms (e.g., genetic variants, extracellular RNA, proteins, lipids, and metabolites). Importantly, this dataset will be available to the public for future research analyses. Using a large dataset that includes data for both men and women will allow future studies to examine for sex-specific differences in mechanisms of chronic pain to generate novel hypotheses.
-
Acknowledgments
The authors have received research support through the NIH including: U24NS112873 (KAS), R01AR077418 (KAS), R01AR073187 (KAS), U24NS112873-03S2 (GB), T32NS045549 (AFS).
Address correspondence to: Kathleen A. Sluka, Roy J Carver Chair in Neuroscience, 500 Newton Road, 1-252 Medical Education Building, Iowa City, Iowa 52242-1089, USA. Email: kathleen-sluka@uiowa.edu.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Smith et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(11):e191931. https://doi.org/10.1172/JCI191931.
-
References
- Katzman JG, Gallagher RM. Pain: the silent public health epidemic. J Prim Care Community Health. 2024;15:21501319241253547.
- Institute of Medicine Committee on Advancing Pain Research Care, Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press; 2011.
- Treede RD, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27.
- Nicholas M, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28–37.
- Schug SA, et al. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52.
- Perrot S, et al. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77–82.
- Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58.
- Queme LF, Jankowski MP. Sex differences and mechanisms of muscle pain. Curr Opin Physiol. 2019;11:1–6.
- Gregus AM, et al. Sex differences in neuroimmune and glial mechanisms of pain. Pain. 2021;162(8):2186–2200.
- Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Sci. 2019;237:116926.
- Alexander SN, et al. The influence of sex on neuroimmune communication, pain, and physiology. Biol Sex Differ. 2024;15(1):82.
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866.
- Fillingim RB, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485.
- Riley JL, et al. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2-3):181–187.
- Stubbs D, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med. 2010;11(2):232–239.
- Glass N, et al. Examining sex differences in knee pain: the multicenter osteoarthritis study. Osteoarthritis Cartilage. 2014;22(8):1100–1106.
- Tonelli SM, et al. Women with knee osteoarthritis have more pain and poorer function than men, but similar physical activity prior to total knee replacement. Biol Sex Differ. 2011;2:12.
- Cooper NA, et al. Predictors of multidimensional functional outcomes after total knee arthroplasty. J Orthop Res. 2017;35(12):2790–2798.
- Gulati M, et al. The influence of sex hormones on musculoskeletal pain and osteoarthritis. Lancet Rheumatol. 2023;5(4):225–238.
- Watt FE. Musculoskeletal pain and menopause. Post Reprod Health. 2018;24(1):34–43.
- Boerner KE, et al. A developmental framework for understanding the influence of sex and gender on health: Pediatric pain as an exemplar. Neurosci Biobehav Rev. 2024;158:105546.
- Lund CI, et al. How is age at menopause and reproductive lifespan associated with chronic pain outcomes in postmenopausal women? Pain. 2025;166(1):144–152.
- Averitt DL, et al, eds. Role of Sex Hormones on Pain. Oxford University Press; 2019.
- Lesnak JB, et al. Testosterone protects against the development of widespread muscle pain in mice. Pain. 2020;161(12):2898–2908.
- Tennant F, Lichota L. Traditional chinese medicine for fibromyalgia. Pract Pain Manag. 2010;10(7):12. View this article via: PubMed Google Scholar
- Athnaiel O, et al. The role of sex hormones in pain-related conditions. Int J Mol Sci. 2023;24(3):1866.
- Craft RM, et al. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411.
- Tengstrand B, et al. Gonadal hormones in men with rheumatoid arthritis--from onset through 2 years. J Rheumatol. 2009;36(5):887–892.
- Pikwer M, et al. Association between testosterone levels and risk of future rheumatoid arthritis in men: a population-based case-control study. Ann Rheum Dis. 2014;73(3):573–579.
- White HD, et al. Treatment of pain in fibromyalgia patients with testosterone gel: Pharmacokinetics and clinical response. Int Immunopharmacol. 2015;27(2):249–256.
- Cheng L, Wang S. Lower serum testosterone is associated with increased likelihood of arthritis. Sci Rep. 2023;13(1):19241.
- Amandusson Å, Blomqvist A. Estrogenic influences in pain processing. Front Neuroendocrinol. 2013;34(4):329–349.
- Kozinoga M, et al. Low back pain in women before and after menopause. Prz Menopauzalny. 2015;14(3):203–207.
- Niesters M, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151(1):61–68.
- Pisanu C, et al. Sex differences in the response to opioids for pain relief: A systematic review and meta-analysis. Pharmacol Res. 2019;148:104447.
- Sharp JL, et al. Sex differences in opioid receptor mediated effects: Role of androgens. Neurosci Biobehav Rev. 2022;134:104522.
- Boerner KE, et al. Sex differences in the efficacy of psychological therapies for the management of chronic and recurrent pain in children and adolescents: A systematic review and meta-analysis. Pain. 2017;258(4):569–582.
- Flegge LG, et al. Sex differences in interdisciplinary pain rehabilitation outcomes: a systematic review. Scand J Pain. 2022;22(2):218–231.
- Hooten WM, et al. Gender differences among patients with fibromyalgia undergoing multidisciplinary pain rehabilitation. Pain Med. 2007;8(8):624–632.
- Keogh E, et al. Do men and women differ in their response to interdisciplinary chronic pain management? Pain. 2005;114(1-2):37–46.
- Murphy JL, et al. Sex differences between Veterans participating in interdisciplinary chronic pain rehabilitation. J Rehabil Res Dev. 2016;53(1):83–94.
- Pieh C, et al. Gender differences in outcomes of a multimodal pain management program. Pain. 2012;153(1):197–202.
- Porreca F, et al. Evaluation of outcomes of calcitonin gene-related peptide (CGRP)-targeting therapies for acute and preventive migraine treatment based on patient sex. Cephalalgia. 2024;44(3):3331024241238153.
- Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014;259:57–63.
- El-Metwally A, et al. Lower limb pain in a preadolescent population: prognosis and risk factors for chronicity--a prospective 1- and 4-year follow-up study. Pediatrics. 2005;116(3):673–681.
- Chidambaran V, et al. DNA methylation at the mu-1 opioid receptor gene (OPRM1) promoter predicts preoperative, acute, and chronic postsurgical pain after spine fusion. Pharmgenomics Pers Med. 2017;10:157–168.
- Rabbitts JA, et al. Prevalence and predictors of chronic postsurgical pain in children: a systematic review and meta-analysis. J Pain. 2017;18(6):605–614.
- Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–744.
- Noiseux NO, et al. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty. 2014;29(7):1383–1387.
- Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15(9):887–897.
- Wandner LD, et al. NIH’s helping to end addiction long-termSM initiative (NIH HEAL Initiative) clinical pain management common data element program. J Pain. 2022;23(3):370–378.
- Sluka KA, et al. Predicting chronic postsurgical pain: current evidence and a novel program to develop predictive biomarker signatures. Pain. 2023;164(9):1912–1926.
- Schwaller F, Fitzgerald M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur J Neurosci. 2014;39(3):344–352.
- Maleki N, et al. At the intersection of alcohol use disorder and chronic pain. Neuropsychology. 2019;33(6):795–807.
- Zale EL, et al. Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev. 2015;37:57–71.
- Ong AD, et al. Positive affect and chronic pain: a preregistered systematic review and meta-analysis. Pain. 2020;161(6):1140–1149.
- Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain. 2015;31(2):177–187.
- Hanssen MM, et al. Can positive affect attenuate (persistent) pain? State of the art and clinical implications. Curr Rheumatol Rep. 2017;19(12):80.
- Sturgeon JA, Zautra AJ. Resilience: a new paradigm for adaptation to chronic pain. Curr Pain Headache Rep. 2010;14(2):105–112.
- Kinnie KR, et al. Chronic pain resilience across clinical populations: a concept analysis. Pain Manag Nurs. 2024;25(5):442–450.
- Bauer H, et al. Resilience moderates the association between chronic pain and depressive symptoms in the elderly. Eur J Pain. 2016;20(8):1253–1265.
- Apkarian AV, et al. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26(4):360–367.
- Baliki MN, et al. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160.
- Baliki MN, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119.
- Mansour AR, et al. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154(10):2160–2168.
- Musto AE, et al. Hippocampal neuro-networks and dendritic spine perturbations in epileptogenesis are attenuated by neuroprotectin d1. PLoS One. 2015;10(1):e0116543.
- Vachon-Presseau E, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(pt 7):1958–1970.
- Petersen KK, et al. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain. 2021;162(1):31–44.
- Paredes AC, et al. Predictive value of quantitative sensory testing for acute and chronic postsurgical pain after total joint arthroplasty: a systematic review. Pain. 2022;163(3):e385–e400.
- He BH, et al. Genetic risk factors for chronic postsurgical pain in children: A narrative review. Eur J Anaesthesiol. 2023;40(7):472–483.
- Chidambaran V, et al. Systematic review and meta-analysis of genetic risk of developing chronic postsurgical pain. J Pain. 2020;21(1-2):2–24.
- Diaz MM, et al. Toward composite pain biomarkers of neuropathic pain-focus on peripheral neuropathic pain. Front Pain Res (Lausanne). 2022;3:869215.
- Mackey S, et al. Neuroimaging-based pain biomarkers: definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. Pain Rep. 2019;4(4):e762.
- Zebhauser PT, et al. Resting-state electroencephalography and magnetoencephalography as biomarkers of chronic pain: a systematic review. Pain. 2023;164(6):1200–1221.
- Hall OT, et al. Central sensitization in opioid use disorder: a novel application of the american college of rheumatology fibromyalgia survey criteria. Pain Rep. 2022;7(4):e1016.
- Schaffer J, et al. Chronic pain, chronic stress and substance use: overlapping mechanisms and implications. Front Pain Res (Lausanne). 2023;4:1145934.
- Linnstaedt SD, et al. A functional riboSNitch in the 3’ untranslated region of FKBP5 alters MicroRNA-320a binding efficiency and mediates vulnerability to chronic post-traumatic pain. J Neurosci. 2018;38(39):8407–8420.
- Trevino CM, et al. Relationship between decreased cortisol and development of chronic pain in traumatically injured. J Surg Res. 2022;270:286–292.
- Plumb AN, et al. Standing on the shoulders of bias: lack of transparency and reporting of critical rigor characteristics in pain research. Pain. 2023;164(8):1775–1782.
- Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21(7):353–365.
- Stevans JM, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4(2):e2037371.
- McLean SA, et al. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain. 2014;155(2):309–321.
- Chang KY, et al. Factors affecting patient-controlled analgesia requirements. J Formos Med Assoc. 2006;105(11):918–925.
- Peters ML, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg. 2007;245(3):487–494.
- Kalkman JC, et al. Preoperative prediction of severe postoperative pain. Pain. 2003;105(3):415–423.
- Chung F, et al. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85(4):808–816.
- King S, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738.
- Pagé MG, et al. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–180.
- Connelly M, et al. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2014;39(3):E174–E181.
- Narayanasamy S, et al. Pediatric pain screening tool: a simple 9-item questionnaire predicts functional and chronic postsurgical pain outcomes after major musculoskeletal surgeries. J Pain. 2022;23(1):98–111.
- Thomas T, et al. Prediction and assessment of the severity of post-operative pain and of satisfaction with management. Pain. 1998;75(2-3):177–185.
- Le LHL, et al. Sex differences in pain catastrophizing and its relation to the transition from acute pain to chronic pain. BMC Anesthesiol. 2024;24(1):127.
- Roca G, et al. Sex differences in chronic postsurgical pain after open thoracotomy. J Cardiothorac Vasc Anesth. 2024;38(12):3134–3142.
- Chaloner A, Greenwood-Van Meerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. 2013;7:13.
- Gong WY, et al. Resident macrophages in muscle contribute to development of hyperalgesia in a mouse model of noninflammatory muscle pain. J Pain. 2016;17(10):1081–1094.
- Gregory NS, et al. Fatigue-enhanced hyperalgesia in response to muscle insult: induction and development occur in a sex-dependent manner. Pain. 2013;154(12):2668–2676.
- Aley KO, et al. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685.
- Severino A, et al. Mu-opioid receptors in nociceptive afferents produce a sustained suppression of hyperalgesia in chronic pain. Pain. 2018;159(8):1607–1620.
- Araldi D, et al. Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain. 2017;158(7):1204–1216.
- DeSantana JM, et al. Animal models of fibromyalgia. Arthritis Res Ther. 2013;15(6):222.
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–618.
- Avona A, et al. Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger. Pain. 2020;161(11):2539–2550.
- Shi X, et al. Sestrin2 prevents neonatal incision pain and re-incision enhanced hyperalgesia in adult rats. Brain Res. 2023;1805:148287.
- Baumbach JL, et al. Inflammatory injury induces pain sensitization that is expressed beyond the site of injury in male (and not in female) mice. Behav Brain Res. 2024;475:115215.
- Joseph EK, et al. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105(1-2):143–150.
- Paige C, et al. Neuroendocrine mechanisms governing sex differences in hyperalgesic priming involve prolactin receptor sensory neuron signaling. J Neurosci. 2020;40(37):7080–7090.
- Megat S, et al. A critical role for dopamine D5 receptors in pain chronicity in male mice. J Neurosci. 2018;38(2):379–397.
- Baptista-de-Souza D, et al. Sex differences in the role of atypical PKC within the basolateral nucleus of the amygdala in a mouse hyperalgesic priming model. Neurobiol Pain. 2020;8:100049.
- Plumb AN, et al. Pregabalin produces analgesia in males but not females in an animal model of chronic widespread muscle pain. Pain Rep. 2024;9(6):e1207.
- Chang JH, et al. Ovarian hormone-dependent and spinal ERK activation-regulated nociceptive hypersensitivity in female rats with acid injection-induced chronic widespread muscle pain. Sci Rep. 2019;9(1):3077.
- Sluka KA, et al. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24(1):37–46.
- Quijas MM, et al. Sex-specific role of RNA-binding protein, pAUF1, on prolonged hypersensitivity after repetitive ischemia with reperfusion injury. Pain. 2025;166(3):693–707.
- Hu YY, et al. Glucocorticoid signaling mediates stress-induced migraine-like behaviors in a preclinical mouse model. Cephalalgia. 2024;44(8):3331024241277941.
- Green PG, et al. Further validation of a model of fibromyalgia syndrome in the rat. J Pain. 2011;12(7):811–818.
- Singaravelu SK, et al. Rat dorsal horn neurons primed by stress develop a long-lasting manifest sensitization after a short-lasting nociceptive low back input. Pain Rep. 2021;6(1):e904.
- LaPrairie JL, Murphy AZ. Long-term impact of neonatal injury in male and female rats: Sex differences, mechanisms and clinical implications. Front Neuroendocrinol. 2010;31(2):193–202.
- Low LA, Fitzgerald M. Acute pain and a motivational pathway in adult rats: influence of early life pain experience. PLoS One. 2012;7(3):e34316.
- Alvarez P, et al. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiatry. 2013;74(9):688–695.
- Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5(1):35–50.
- Brewer CL, Baccei ML. The development of pain circuits and unique effects of neonatal injury. J Neural Transm (Vienna). 2020;127(4):467–479.
- Cooper AH, et al. Neonatal complete Freund’s adjuvant-induced inflammation does not induce or alter hyperalgesic priming or alter adult distributions of C-fibre dorsal horn innervation. Pain Rep. 2020;5(6):e872.
- Page GG, et al. Sex differences in pain responses at maturity following neonatal repeated minor pain exposure in rats. Biol Res Nurs. 2013;15(1):96–104.
- Dourson AJ, et al. Macrophage memories of early-life injury drive neonatal nociceptive priming. Cell Rep. 2024;43(5):114129.
- Moriarty O, et al. Priming of adult incision response by early-life injury: neonatal microglial inhibition has persistent but sexually dimorphic effects in adult rats. J Neurosci. 2019;39(16):3081–3093.
- Knaepen L, et al. Sex differences in inflammatory mechanical hypersensitivity in later life of rats exposed to repetitive needle pricking as neonates. Neurosci Lett. 2012;516(2):285–289.
- Bertagna NB, et al. Long-lasting mechanical hypersensitivity and CRF receptor type-1 neuron activation in the BNST following adolescent ethanol exposure. Alcohol Clin Exp Res (Hoboken). 2024;48(1):48–57.
- Marvizon JC, et al. Latent sensitization: a model for stress-sensitive chronic pain. Curr Protoc Neurosci. 2015;71:9.50.1–9.50.14.
- Taylor BK, Corder G. Endogenous analgesia, dependence, and latent pain sensitization. Curr Top Behav Neurosci. 2014;20:283–325.
- Inyang KE, et al. The μ-δ opioid heteromer masks latent pain sensitization in neuropathic and inflammatory pain in male and female mice. Brain Res. 2021;1756:147298.
- Custodio-Patsey L, et al. Sex differences in kappa opioid receptor inhibition of latent postoperative pain sensitization in dorsal horn. Neuropharmacology. 2020;163:107726.
- Lesnak JB, et al. Influence of routine exercise on the peripheral immune system to prevent and alleviate pain. Neurobiol Pain. 2023;13:100126.
- Tong SH, et al. Emerging role of macrophages in neuropathic pain. J Orthop Translat. 2025;51:227–241.
- Khomula EV, et al. Sexual dimorphism in a reciprocal interaction of ryanodine and IP3 receptors in the induction of hyperalgesic priming. J Neurosci. 2017;37(8):2032–2044.
- Ferrari LF, et al. Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33(27):11002–11011.
- Ferrari LF, et al. Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep. 2016;6(1):31221.
- Ferrari LF, et al. Second messengers mediating the expression of neuroplasticity in a model of chronic pain in the rat. J Pain. 2014;15(3):312–320.
- Chang JH, et al. Role of ERK in gender difference of fibromyalgia pain. Mol Pain. 2024;20:17448069241261940.
- Hayashi K, et al. Brain-derived neurotrophic factor contributes to activity-induced muscle pain in male but not female mice. Brain Behav Immun. 2024;120:471–487.
- Moy JK, et al. Temporal and sex differences in the role of BDNF/TrkB signaling in hyperalgesic priming in mice and rats. Neurobiol Pain. 2019;5:100024.
- Hayashi K, et al. P2X7-NLRP3-Caspase-1 signaling mediates activity-induced muscle pain in male but not female mice. Pain. 2023;164(8):1860–1873.
- Gregory NS, et al. ASIC3 is required for development of fatigue-induced hyperalgesia. Mol Neurobiol. 2016;53(2):1020–1030.
- Lesnak JB, et al. The impact of sex and physical activity on the local immune response to muscle pain. Brain Behav Immun. 2023;111:4–20.
- Oliveira-Fusaro MC, et al. P2X4 receptors on muscle macrophages are required for development of hyperalgesia in an animal model of activity-induced muscle pain. Mol Neurobiol. 2020;57(4):1917–1929.
- Brito RG, et al. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain Rep. 2017;2(5):e618.
- Lesnak JB, et al. Resistance training protects against muscle pain through activation of androgen receptors in male and female mice. Pain. 2022;163(10):1879–1891.
- Leung A, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606.
- Sabharwal R, et al. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016;157(2):387–398.
- Bobinski F, et al. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain. 2018;159(3):437–450.
- Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129.
- Nasir H, et al. Consistent sex-dependent effects of PKMζ gene ablation and pharmacological inhibition on the maintenance of referred pain. Mol Pain. 2016;12:1744806916675347.
- Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23(13):5437–5445.
- Chen WH, et al. Spinal protein kinase C/extracellular signal-regulated kinase signal pathway mediates hyperalgesia priming. Pain. 2018;159(5):907–918.
- Basu P, et al. Sex differences in protein kinase a signaling of the latent postoperative pain sensitization that is masked by kappa opioid receptors in the spinal cord. J Neurosci. 2021;41(47):9827–9843.
- Changsheng L, et al. Role of descending dopaminergic pathways in pain modulation. Curr Neuropharmacol. 2019;17(12):1176–1182.
- Kim JY, et al. Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism. J Neurosci. 2015;35(16):6307–6317.
- Li J, Baccei ML. Neonatal tissue damage promotes spike timing-dependent synaptic long-term potentiation in adult spinal projection neurons. J Neurosci. 2016;36(19):5405–5416.
- Li J, et al. D1/D5 dopamine receptors and mGluR5 jointly enable non-hebbian long-term potentiation at sensory synapses onto lamina i spinoparabrachial neurons. J Neurosci. 2022;42(3):350–361.
- Tansley S, et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat Commun. 2022;13(1):843.
- Ghazisaeidi S, et al. Neuropathic pain: mechanisms, sex differences, and potential therapies for a global problem. Annu Rev Pharmacol Toxicol. 2023;63:565–583.
- Huck NA, et al. Temporal contribution of myeloid-lineage TLR4 to the transition to chronic pain: a focus on sex differences. J Neurosci. 2021;41(19):4349–4365.
- Mogil JS, et al. Sex differences in mechanisms of pain hypersensitivity. Neurosci Biobehav Rev. 2024;163:105749.
- Paige C, et al. Spinal inhibition of P2XR or p38 signaling disrupts hyperalgesic priming in male, but not female, mice. Neuroscience. 2018;385:133–142.
- Cooper AH, et al. Endogenous μ-opioid receptor activity in the lateral and capsular subdivisions of the right central nucleus of the amygdala prevents chronic postoperative pain. J Neurosci Res. 2022;100(1):48–65.
- Lin Y-L, et al. Cellular mechanisms underlying central sensitization in a mouse model of chronic muscle pain. Elife. 2022;11:e78610.
- Chen W-K, et al. Ca(v)3.2 T-type Ca2+ channel-dependent activation of ERK in paraventricular thalamus modulates acid-induced chronic muscle pain. J Neurosci. 2010;30(31):10360–10368.
- Athnaiel O, et al. Gonadal hormone changes with aging and their impact on chronic pain. Cells. 2025;14(2):123.
- Erceg N, et al. The role of cortisol and dehydroepiandrosterone in obesity, pain, and aging. Diseases. 2025;13(2):42.
- Plumb AN, et al. Local synthesis of estradiol in the rostral ventromedial medulla protects against widespread muscle pain in male mice. eNeuro. 2024;11(8):ENEURO.0332-24.2024.
- Plumb AN, et al. Female specific interactions of serotonin and testosterone in the rostral ventromedial medulla after activity-induced muscle pain. J Pain. 2024;26:104723.
- Eller-Smith OC, et al. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci. 2018;12:35.
- Staurengo-Ferrari L, et al. Neuroendocrine mechanisms in oxaliplatin-induced hyperalgesic priming. Pain. 2023;164(6):1375–1387.
- Alvarez P, et al. Neonatal handling (resilience) attenuates water-avoidance stress induced enhancement of chronic mechanical hyperalgesia in the rat. Neurosci Lett. 2015;591:207–211.
- Keogh E, Boerner KE. Challenges with embedding an integrated sex and gender perspective into pain research: Recommendations and opportunities. Brain Behav Immun. 2024;117:112–121.
- Palermo TM, et al. Promoting inclusion, diversity and equity in pain science. Eur J Pain. 2023;27(4):451–456.
- Sluka KA, et al. Predicting chronic postsurgical pain: current evidence and a novel program to develop predictive biomarker signatures. Pain. 2023;164(9):1912–1926.
-
Version history
- Version 1 (June 2, 2025): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Metrics
Go to
- Top
- Abstract
- Introduction
- Sex differences in pain
- Acute-to-chronic–pain transition incidence and predictors
- Sex-specific differences in the transition to chronic pain
- Animal models of the transition to chronic pain
- Peripheral neural and immune mechanisms in animal models
- Central neural and immune mechanisms in animal models
- Endocrine mechanisms in animals
- Sex hormones
- HPA axis
- Outlook on sex-dependent factors in chronic pain
- Supplemental material
- Acknowledgments
- Footnotes
- References
- Version history



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)



