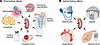Issue published November 3, 2025 Previous issue

- Volume 135, Issue 21
Go to section:
On the cover: Linking severe systemic inflammation to vascular dysfunction
Martino et al. report that the inflammatory cytokine IL-6 drives endothelial stress by promoting transient mitochondrial dysfunction and DNA release into the cytoplasm, leading to direct activation of cGAS and STING. Image credit: Fatma Awadalla and Alejandro P. Adam.
-
Editorial
Elizabeth M. McNally, … , Corinne L. Williams, Oliver EickelbergElizabeth M. McNally, … , Corinne L. Williams, Oliver EickelbergPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e201281. https://doi.org/10.1172/JCI201281.×Abstract
Authors
Elizabeth M. McNally, Sarah Jackson, Corinne L. Williams, Oliver Eickelberg
-
Review Series
Estefania Valencia-Rincón, … , Vishal Chandra, Elizabeth A. WellbergEstefania Valencia-Rincón, … , Vishal Chandra, Elizabeth A. WellbergPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e194743. https://doi.org/10.1172/JCI194743.×Abstract
Cancer diagnoses are prevalent in people with obesity and type 2 diabetes, and abundant clinical evidence supports the protective effects of weight loss for cancer prevention. Glucagon-like peptide-1 (GLP-1) receptor agonists have revolutionized obesity and type 2 diabetes medicine and alleviate many comorbidities of these metabolic diseases. In this Review, we summarize the current clinical evidence for GLP-1 receptor agonists and cancer risk, including thyroid, pancreatic, gastrointestinal, and hormone-dependent malignancies. With few exceptions, recent meta-analyses report that GLP-1 receptor therapies do not increase cancer incidence and may lower risk in some cases. Preclinical studies reinforce the anticancer effects of GLP-1 receptor therapies, even in non-obese models. However, there are still many opportunities for translational insight as the field grows. Immune-modulating effects of GLP-1 receptor agonists are reported in several preclinical cancer studies, which may reflect direct action on immune cells or result from improved metabolic function. We highlight ongoing clinical trials for GLP-1 receptor therapies in cancer patients, and offer considerations for preclinical studies, including perspectives on the timing and duration of GLP-1 receptor agonist treatment, concurrent use of standard anticancer therapies, and interpretation of models of cancer risk versus progression.
Authors
Estefania Valencia-Rincón, Rajani Rai, Vishal Chandra, Elizabeth A. Wellberg
Mark E. Cooper, Daniël H. van RaalteMark E. Cooper, Daniël H. van RaaltePublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e194749. https://doi.org/10.1172/JCI194749.×Abstract
Glucagon-like peptide-1 (GLP-1) was initially considered to be a hormone with a predominant role in regulating glucose metabolism by inducing insulin secretion, reducing glucagon secretion, and ameliorating insulin resistance, with the last effect being largely dependent on the induction of weight loss. In more recent years, the role of this peptide beyond metabolism has progressively been explored, including its impact on kidney physiology and kidney clinical outcomes in people with obesity with or without diabetes. Indeed, despite only modest expression of the GLP-1 receptor in the kidney, the renoprotective actions of GLP-1 and its receptor agonists have become an area of intensive investigation. This Review appraises the current status of GLP-1 peptide and its receptor agonists and focuses on the preclinical as well as recent seminal clinical findings defining the kidney benefits conferred by GLP-1 receptor agonist treatment in people living with type 2 diabetes and obesity.
Authors
Mark E. Cooper, Daniël H. van Raalte
Chi Kin Wong, Daniel J. DruckerChi Kin Wong, Daniel J. DruckerPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e194751. https://doi.org/10.1172/JCI194751.×Abstract
Therapies based on glucagon-like peptide-1 (GLP-1) reduce rates of cardiovascular and chronic kidney disease in people with type 2 diabetes and/or obesity, with ongoing clinical trials investigating their effects in people with metabolic liver disease, arthritis, and both substance use and neurodegenerative disorders. Acute and chronic activation of GLP-1 receptor signaling also reduces systemic and tissue inflammation in mice and humans, through weight loss–dependent and –independent mechanisms, actions that may contribute to the expanding spectrum of clinical benefits ascribed to GLP-1 medicines. In this Review, we highlight current understanding of the direct and indirect antiinflammatory effects and mechanisms of GLP-1 medicines in both preclinical and clinical studies, covering emerging concepts, clinical relevance, and areas of uncertainty that require further investigation.
Authors
Chi Kin Wong, Daniel J. Drucker
-
Review
Caglar Cosarderelioglu, Peter M. AbadirCaglar Cosarderelioglu, Peter M. AbadirPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e195633. https://doi.org/10.1172/JCI195633.×Abstract
The renin-angiotensin-aldosterone system (RAAS) is a central regulator of cardiovascular, renal, and fluid homeostasis. Over the past century, our understanding of RAAS has evolved from a unidimensional circulatory hormone system to a complex network that includes local and intracellular signaling pathways. Aging profoundly impacts this system, influencing both systemic and tissue-specific RAAS activity. While levels of systemic RAAS components, such as plasma renin and aldosterone, decline with age, local RAAS components, particularly the proinflammatory angiotensin (Ang)II/AngII type 1 receptor (AT1R) axis, are upregulated in aging tissues, contributing to vasoconstriction, oxidative stress, inflammation, and fibrosis. Conversely, the protective arms of RAAS, the AngII/AT2R and Ang-(1–7)/Mas receptor pathways, are downregulated. Recent advances in geroscience have further illuminated how RAAS intersects with fundamental aging mechanisms, providing a mechanistic framework for understanding RAAS not only as a driver of age-related disease but also as a modifiable contributor to the aging process itself. In this Review, we summarize the evolution of RAAS biology, examine the molecular and functional consequences of aging on RAAS activity, and discuss the translational relevance of these findings. Finally, we explore emerging therapeutic strategies targeting RAAS components as potential interventions to promote healthy aging and reduce age-related disease burden, emphasizing a translational arc moving from bedside to bench and back, with the ultimate goal of improving patient outcomes.
Authors
Caglar Cosarderelioglu, Peter M. Abadir
-
Editor’s note
G.R. Scott BudingerG.R. Scott BudingerPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e200729. https://doi.org/10.1172/JCI200729.×Abstract
Authors
G.R. Scott Budinger
-
Commentaries
Richard F. Loeser, Philip R. CoryellRichard F. Loeser, Philip R. CoryellPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e197144. https://doi.org/10.1172/JCI197144.×Abstract
Osteoarthritis (OA) is a highly prevalent and painful joint disease in desperate need of disease-modifying therapeutics. Decline in the activity of the Forkhead box O (FOXO) family of transcriptional regulators in articular chondrocytes may contribute to the development of OA. In a study in this issue of the JCI, Kurakazu et al. screened compounds for FOXO activators and discovered that the antihistamine cyproheptadine activated FOXO3 through inhibition of the histamine H1 receptor. Cyproheptadine modulated the activity of OA-relevant pathways and reduced the severity of joint damage and pain behavior in a mouse model of OA, thus showing potential for development as a disease-modifying OA drug.
Authors
Richard F. Loeser, Philip R. Coryell
Amanda Luvisotto, Lu WangAmanda Luvisotto, Lu WangPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e198684. https://doi.org/10.1172/JCI198684.×Abstract
In acute myeloid leukemia (AML), leukemogenesis is typically driven by the sequential acquisition of distinct classes of mutations that collaborate to transform normal hematopoietic stem and progenitor cells. The founding and cooperating mutations in AML are often in signaling genes and form functional partnerships with each other, each addressing complementary aspects of malignant transformation. In this issue of the JCI, Kramer et al. elaborate on the molecular pathogenesis of AML. By using a mouse bone marrow model bearing the common AML-initiating mutations in DNA methyltransferase 3 α (DNMT3A) and nucleophosmin 1 (NPM1), the work provides further evidence for the role of the signaling orchestrator GRB2-associated–binding protein 2 (GAB2) in AML progression, positioning GAB2 as a potential therapeutic target.
Authors
Amanda Luvisotto, Lu Wang
Shojiro Haji, Yoshihiro OgawaShojiro Haji, Yoshihiro OgawaPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e198802. https://doi.org/10.1172/JCI198802.×Abstract
Increased activation of the NLRP3 inflammasome in immune cells, including macrophages, has been implicated in the pathogenesis of multiple chronic inflammatory diseases. Targeted depletion of macrophages has been explored as a cross-disease therapeutic strategy, but without subtype-specific markers, this strategy risks elimination of macrophages with homeostatic functions. In this study, Liu et al. identified a subpopulation of pathogenic macrophages, referred to as Toe-Macs, which are characterized by overexpression of the DNA demethylase TET3 in metabolic dysfunction–associated steatohepatitis (MASH), non–small cell lung cancer (NSCLC), and endometriosis. When induced into the disease microenvironment, Toe-Macs produced proinflammatory cytokines and chemokines. Selective elimination of Toe-Macs attenuated disease progression without any discernible side effects in mouse models of MASH and NSCLC. These findings highlight the role of Toe-Macs in the pathogenesis of chronic inflammatory diseases and provide a rationale for exploring TET3 as a therapeutic target.
Authors
Shojiro Haji, Yoshihiro Ogawa
Roser Tachó-Piñot, Carola G. VinuesaRoser Tachó-Piñot, Carola G. VinuesaPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e198981. https://doi.org/10.1172/JCI198981.×Abstract
In systemic lupus erythematosus (SLE), autoimmunity often develops toward self nucleic acids. The nucleic acid receptors TLR7 and TLR9, which sense RNA and DNA, respectively, are critical for the generation of pathogenic autoimmune antibodies. Despite similarities in their downstream signaling cascades, these receptors play opposing roles in most mouse lupus models: TLR7 promotes disease, while TLR9 provides protection — an observation often referred to as “the TLR paradox.” To understand the basis of this dichotomy, Leibler et al. created genetically edited lupus-prone mice in which TLR7 receptors express the TLR intracellular signaling domain (TIR) that corresponds to TLR9, or vice versa. Their results revealed that the TIR domains contribute to the receptors’ opposing roles in SLE, shedding light into the TLR paradox in autoimmunity.
Authors
Roser Tachó-Piñot, Carola G. Vinuesa
Ajay Tambralli, Jason S. KnightAjay Tambralli, Jason S. KnightPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e199299. https://doi.org/10.1172/JCI199299.×Abstract
Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome is an adult-onset inflammatory disorder caused by somatic UBA1 mutations in hematopoietic stem cells. UBA1 encodes a key enzyme that catalyzes protein ubiquitination. Clinically, VEXAS is characterized by systemic inflammation and hematologic abnormalities. Patient studies have hinted that the transition of UBA1-mutated stem cells into proinflammatory myeloid precursors may propagate the manifestations of VEXAS syndrome. In this issue of the JCI, Dong and colleagues developed nine unique conditional knockout mouse strains and found that only neutrophil-specific Uba1 deletion reproduced VEXAS syndrome–like findings. The observed phenotype was at least in part due to inflammatory reprogramming and longer survival of the mutant neutrophils. In addition to deepening our mechanistic understanding of VEXAS syndrome pathogenesis, this work should provide a platform to pursue more targeted approaches to treatment.
Authors
Ajay Tambralli, Jason S. Knight
-
Research Letters
Kristy Tefft, … , Brett King, Jaehyuk ChoiKristy Tefft, … , Brett King, Jaehyuk ChoiPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e190853. https://doi.org/10.1172/JCI190853.×Abstract
Authors
Kristy Tefft, Amy Wang, Zachary Z. Reinstein, Yue Zhang, Arundhati Pillai, Sunghee Hwang, Spencer Ng, Raymond J. Cho, Jeffrey B. Cheng, Fei Li Kuang, Brett King, Jaehyuk Choi
Mindy K. Graham, Sarki A. AbdulkadirMindy K. Graham, Sarki A. AbdulkadirPublished August 28, 2025
Citation Information: J Clin Invest. 2025;135(21):e196347. https://doi.org/10.1172/JCI196347.×Abstract
Authors
Mindy K. Graham, Sarki A. Abdulkadir
Masahiko Shigemura, … , Jacob I. Sznajder, Ankit BharatMasahiko Shigemura, … , Jacob I. Sznajder, Ankit BharatPublished August 26, 2025
Citation Information: J Clin Invest. 2025;135(21):e196928. https://doi.org/10.1172/JCI196928.×Abstract
Authors
Masahiko Shigemura, Felix L. Nuñez Santana, S. Marina Casalino-Matsuda, David Kirchenbuechler, Radmila Nafikova, Fei Chen, Zhan Yu, Yuliana V. Sokolenko, Estefani Diaz, Suchitra Swaminathan, Suror Mohsin, Rizaldy P. Scott, Lynn C. Welch, Chitaru Kurihara, Emilia Lecuona, G.R. Scott Budinger, Peter H.S. Sporn, Jacob I. Sznajder, Ankit Bharat
-
Research Articles
Emilie Crouchet, … , Thomas F. Baumert, Catherine SchusterEmilie Crouchet, … , Thomas F. Baumert, Catherine SchusterPublished September 11, 2025
Citation Information: J Clin Invest. 2025;135(21):e169395. https://doi.org/10.1172/JCI169395.×Abstract
Treatment options for advanced liver disease and hepatocellular carcinoma (HCC) are limited, and strategies to prevent HCC development are lacking. Aiming to discover therapeutic targets, we combined genome-wide transcriptomic analysis of liver tissues from patients with advanced liver disease and HCC and a cell-based system predicting liver disease progression and HCC risk. Computational analysis predicted peroxiredoxin 2 (PRDX2) as a candidate gene mediating hepatocarcinogenesis and HCC risk. Analysis of tissues from patients with HCC confirmed a perturbed expression of PRDX2 in cancer. In vivo perturbation studies in mouse models for hepatocarcinogenesis driven by metabolic dysfunction–associated steatohepatitis showed that specific Prdx2 KO in hepatocytes improved metabolic liver functions, restored AMPK activity, and prevented HCC development by suppressing oncogenic signaling. Perturbation studies in HCC cell lines, a cell line–derived xenograft mouse model, and patient-derived HCC spheroids revealed that PRDX2 also mediates cancer initiation, cancer cell proliferation, and survival through its antioxidant activity. Targeting PRDX2 may therefore be a strategy to prevent HCC development in metabolic liver disease.
Authors
Emilie Crouchet, Eugénie Schaeffer, Marine A. Oudot, Julien Moehlin, Cloé Gadenne, Frank Jühling, Hussein El Saghire, Naoto Fujiwara, Shijia Zhu, Fahmida Akter Rasha, Sarah C. Durand, Anouk Charlot, Clara Ponsolles, Romain Martin, Nicolas Brignon, Fabio Del Zompo, Laura Meiss-Heydmann, Marie Parnot, Nourdine Hamdane, Danijela Heide, Jenny Hetzer, Mathias Heikenwälder, Emanuele Felli, Patrick Pessaux, Nathalie Pochet, Joffrey Zoll, Brian Cunniff, Yujin Hoshida, Laurent Mailly, Thomas F. Baumert, Catherine Schuster
Heather Blasczyk, … , Nicole Skinner, Christopher M. WalkerHeather Blasczyk, … , Nicole Skinner, Christopher M. WalkerPublished September 16, 2025
Citation Information: J Clin Invest. 2025;135(21):e178089. https://doi.org/10.1172/JCI178089.×Abstract
Sustained CD4+ T cell immunity is required for resolution of acute hepatitis C virus (HCV) infection, but the response remains poorly characterized. Here, circulating CD4+ T cells with high programmed cell death 1 (PD-1) and ICOS coexpression were temporally associated with onset of virus control, seroconversion, and hepatitis in HCV-infected chimpanzees. Coproduction of T follicular helper (Tfh) (IL-21 and CXCL13) and Th1 (IFN-γ and TNF) cytokines after stimulation with HCV nonstructural proteins demonstrated that the response was predominately Tfh1 like and virus specific. Transcriptional analysis verified a Tfh1 lineage assignment. Effector-related genes such as ADGRG1 (GPR56), ZNF683 (Hobit), and KLRB1 (CD161) were also expressed. HCV-specific PD-1hiICOShi CD4+ Tfh1-like cells were enriched in liver, suggesting the potential for B and CD8+ T cell help at the site of virus replication. Most circulating and intrahepatic PD-1hiICOShi CD4+ Tfh1-like cells did not express CXCR5 and therefore resembled CXCR5–CXCL13+ peripheral helper cells that infiltrate tumors and tissues inflamed by autoimmunity. PD-1hiICOShi CD4+ Tfh1-like cells also peaked after hepatitis A virus infection, but the response was accelerated by several weeks compared with HCV infection. The PD-1hiICOShi phenotype and temporal association between the peak response and alanine aminotransferase may provide markers to guide human studies of CD4+ T cell immunity against HCV and other hepatotropic viruses.
Authors
Heather Blasczyk, William Bremer, Christopher C. Phelps, Yan Zhou, David G. Bowen, Zhaohui Xu, Robert Lanford, Naglaa H. Shoukry, Arash Grakoui, Nicole Skinner, Christopher M. Walker
Chun-Yu Fu, … , Milos Filipovic, Guenter SchwarzChun-Yu Fu, … , Milos Filipovic, Guenter SchwarzPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e181299. https://doi.org/10.1172/JCI181299.×Abstract
Sulfite oxidase (SOX) deficiency is a rare inborn error of cysteine metabolism resulting in severe neurological damage. In patients, sulfite accumulates to toxic levels, causing a rise in the downstream products S-sulfocysteine, which mediates excitotoxicity, and thiosulfate, a catabolic intermediate/product of hydrogen sulfide (H2S) metabolism. Here, we report a full-body knockout mouse model for SOX deficiency (SOXD) with a severely impaired phenotype. Among the urinary biomarkers, thiosulfate showed a 45-fold accumulation in SOXD mice, representing the major excreted S-metabolite. Consistently, we found increased plasma H2S, which was derived from sulfite-induced release from persulfides, as demonstrated in vitro and in vivo. Mass spectrometry analysis of total protein persulfidome identified a major loss of S-persulfidation in 20% of the proteome, affecting enzymes in amino acids, fatty acid metabolism, and cytosolic iron-sulfur cluster biogenesis. Urinary amino acid profiles indicated metabolic rewiring and mitochondrial dysfunction, thus identifying an altered H2S metabolism and persulfidation in SOXD. Finally, oxidized glutathione and glutathione trisulfide were able to scavenge sulfite in vitro and in vivo, extending the lifespan of SOXD mice and providing a mechanistic concept of sulfite scavenging for the treatment of this severe metabolic disorder of cysteine catabolism.
Authors
Chun-Yu Fu, Joshua B. Kohl, Filip Liebsch, Davide D’Andrea, Tamás Ditrói, Seiryo Ogata, Franziska Neuser, Max Mai, Anna T. Mellis, Emilia Kouroussis, Masanobu Morita, Titus Gehling, José Angel Santamaria-Araujo, Sin Yuin Yeo, Heike Endepols, Michaela Křížková, Viktor Kozich, Marcus Krueger, Julia B. Hennermann, Uladzimir Barayeu, Takaaki Akaike, Peter Nagy, Milos Filipovic, Guenter Schwarz
Changwoo Lee, … , Janghoo Lim, Andrew P. LiebermanChangwoo Lee, … , Janghoo Lim, Andrew P. LiebermanPublished August 28, 2025
Citation Information: J Clin Invest. 2025;135(21):e182955. https://doi.org/10.1172/JCI182955.×Abstract
Degeneration of the neuromuscular system is a characteristic feature of spinal and bulbar muscular atrophy (SBMA), a CAG/polyglutamine (polyQ) expansion disorder caused by mutation in the androgen receptor (AR). Using a gene-targeted mouse model of SBMA, AR113Q mice, we demonstrate age-dependent degeneration of the neuromuscular system that initially manifests with muscle weakness and atrophy and progresses to include denervation of neuromuscular junctions and lower motor neuron soma atrophy. Using this model, we tested the hypothesis that therapeutic intervention targeting skeletal muscle during this period of disease progression arrests degeneration of the neuromuscular system. To accomplish this, AR-targeted antisense oligonucleotides were administered subcutaneously to symptomatic AR113Q mice to reduce expression of polyQ AR in peripheral tissues but not in the spinal cord. This intervention rescued muscle atrophy, neuromuscular junction innervation, lower motor neuron soma size, and survival in aged AR113Q mice. Single-nucleus RNA sequencing revealed age-dependent transcriptional changes in the AR113Q spinal cord during disease progression, which were mitigated by peripheral AR gene silencing. Our findings underscore the intricate interplay between peripheral tissues and the central nervous system in SBMA and emphasize the therapeutic effectiveness of peripheral gene knockdown in symptomatic disease.
Authors
Changwoo Lee, Zhigang Yu, Curtis J. Kuo, Leon Tejwani, Rosalie M. Grijalva, Eunwoo Bae, Hien T. Zhao, Janghoo Lim, Andrew P. Lieberman
Yi Jiang, … , Jun Wang, Wankun ChenYi Jiang, … , Jun Wang, Wankun ChenPublished September 2, 2025
Citation Information: J Clin Invest. 2025;135(21):e183541. https://doi.org/10.1172/JCI183541.×Abstract
Sepsis is a life-threatening disease caused by a dysfunctional host response to infection. During sepsis, inflammation-related immunosuppression is the critical factor causing secondary infection and multiple organ dysfunction syndrome. The regulatory mechanisms underlying Treg differentiation and function, which significantly contribute to septic immunosuppression, require further clarification. In this study, we found that neutrophil extracellular traps (NETs) participated in the development of sepsis-induced immunosuppression by enhancing Treg differentiation and function via direct interaction with CD4+ T cells. Briefly, NETs anchored enolase 1 (ENO1) on the membrane of CD4+ T cells through its key protein myeloperoxidase (MPO) and subsequently recruited interferon-induced transmembrane protein 2 (IFITM2). IFITM2 acted as a DNA receptor that sensed NET-DNA and activated intracellular RAS-associated protein 1B (RAP1B) and its downstream ERK signaling pathway to promote Treg differentiation and function. ENO1 inhibition significantly attenuated NET-induced Treg differentiation and alleviated sepsis in mice. Overall, we demonstrated the role of NETs in sepsis-induced immunosuppression by enhancing Treg differentiation, identified ENO1 as an anchor of NET-MPO, and elucidated the downstream molecular mechanism by which IFITM2-RAP1B-ERK regulates Treg differentiation. These findings improve our understanding of the immunopathogenesis of sepsis and provide potential therapeutic targets for sepsis-induced immunosuppression.
Authors
Yi Jiang, Shenjia Gao, Xiya Li, Hao Sun, Xinyi Wu, Jiahui Gu, Zhaoyuan Chen, Han Wu, Xiaoqiang Zhao, Tongtong Zhang, Ronen Ben-Ami, Yuan Le, Timothy R. Billiar, Changhong Miao, Jie Zhang, Jun Wang, Wankun Chen
Kohei Kido, … , Jørgen F.P. Wojtaszewski, Rasmus KjøbstedKohei Kido, … , Jørgen F.P. Wojtaszewski, Rasmus KjøbstedPublished September 2, 2025
Citation Information: J Clin Invest. 2025;135(21):e183567. https://doi.org/10.1172/JCI183567.×Abstract
A single bout of exercise improves muscle insulin sensitivity for up to 48 hours via AMPK. Limb ischemia activates AMPK in muscle, and subsequent reperfusion enhances insulin-stimulated vasodilation, potentially eliciting a more pronounced exercise effect with reduced workload. We investigated the combined effect of upper leg intermittent ischemia/reperfusion (IIR) and continuous knee-extension exercise on muscle insulin sensitivity regulation. We found that IIR exercise potentiated AMPK activation and muscle insulin sensitivity. The potentiating effect of IIR exercise on muscle insulin sensitivity was associated with increased insulin-stimulated blood flow in parallel with enhanced phosphorylation of endothelial nitric oxide synthase. Metabolomics analyses demonstrated a suppression of muscle medium-chain acylcarnitines during IIR exercise, which correlated with insulin sensitivity and was consistent with findings in isolated rat muscle treated with decanoyl-l-carnitine. Collectively, combining IIR with low- to moderate-intensity exercise may represent a promising intervention to effectively enhance muscle insulin sensitivity. This approach could offer potential for mitigating muscle insulin resistance in clinical settings and among individuals with lower physical activity levels.
Authors
Kohei Kido, Janne R. Hingst, Johan Onslev, Kim A. Sjøberg, Jesper B. Birk, Nicolas O. Eskesen, Tongzhu Zhou, Kentaro Kawanaka, Jesper F. Havelund, Nils J. Færgeman, Ylva Hellsten, Jørgen F.P. Wojtaszewski, Rasmus Kjøbsted
Ichiro Kurakazu, … , Yasuharu Nakashima, Martin K. LotzIchiro Kurakazu, … , Yasuharu Nakashima, Martin K. LotzPublished August 28, 2025
Citation Information: J Clin Invest. 2025;135(21):e183588. https://doi.org/10.1172/JCI183588.×Abstract
Osteoarthritis (OA) is the most common joint disease. Controlling the complex pathogenesis is challenging, thus, disease-modifying OA drugs are not available. Forkhead box O (FOXO) transcription factors contribute to cartilage homeostasis through autophagy and oxidative stress resistance. Here, we sought to discover FOXO activators and found that cyproheptadine, a histamine H1 receptor (HRH1) inverse agonist, promoted FOXO3 nuclear translocation and increased FOXO target genes while suppressing inflammation. In a murine OA model, cyproheptadine reduced structural joint tissue damage and pain behaviors. Mechanistically, the inhibition of HRH1 constitutive activity mediated the effects of cyproheptadine on calcium balance between endoplasmic reticulum (ER) and cytoplasm, and FOXO activation was part of this mechanism. The antiinflammatory effect of cyproheptadine involved the inhibition of protein kinase C/NF-κB pathway. HRH1 inhibition also suppressed osteogenesis in mesenchymal stem cells and nerve growth factor expression, which are mechanisms of osteophyte formation and pain behaviors. Moreover, cyproheptadine suppressed ER stress–induced lipogenesis by upregulating insulin-induced gene 1. Our findings suggest that HRH1 constitutive activity controls important OA-promoting mechanisms and indicate that HRH1 inverse agonists are promising drug repurposing candidates for structure and pain improvement in OA.
Authors
Ichiro Kurakazu, Merissa Olmer, Hannah Swahn, Kevin Myers, Chelsea Kenvisay, Yukio Akasaki, Yasuharu Nakashima, Martin K. Lotz
Kai Chen, … , Dapeng Chen, Zhixin SongKai Chen, … , Dapeng Chen, Zhixin SongPublished September 2, 2025
Citation Information: J Clin Invest. 2025;135(21):e184721. https://doi.org/10.1172/JCI184721.×Abstract
The persistent challenge of sepsis-related mortality underscores the necessity for deeper insights. Our multicenter, cross-age cohort study identified insulin-like growth factor binding protein 6 (IGFBP6) as a critical regulator in sepsis diagnosis, prognosis, and mortality risk evaluation. Mechanistically, IGFBP6 engages in IGF-independent binding to prohibitin2 (PHB2) on epithelial cells, driving PHB2 tyrosine phosphorylation during sepsis. This process disrupts STAT1 phosphorylation, nuclear translocation, and its recruitment to the CCL2 promoter, ultimately impairing CCL2 transcription and macrophage chemotaxis. Crucially, PHB2 silencing via siPHB2 and STAT1 activation using 2-NP restored CCL2 expression in vitro and in vivo, improving bacterial clearance and survival in septic mice. Concurrently, IGFBP6 compromised macrophage bactericidal activity by inhibiting Akt phosphorylation, reducing ROS/IL-1β production and phagocytic capacity — defects reversible by Akt agonist SC79. Collectively, IGFBP6 emerges as an endogenous driver of sepsis pathogenesis, positioning it as a dual diagnostic biomarker and therapeutic target. Intervention strategies targeting IGFBP6-mediated signaling may offer transformative approaches for sepsis management.
Authors
Kai Chen, Ying Hu, Xiaoyan Yu, Hong Tang, Yanting Ruan, Yue Li, Xun Gao, Qing Zhao, Hong Wang, Xuemei Zhang, David Paul Molloy, Yibing Yin, Dapeng Chen, Zhixin Song
Hayley E. McMorrow, … , Ricardo J. Samms, Lisa R. BeutlerHayley E. McMorrow, … , Ricardo J. Samms, Lisa R. BeutlerPublished August 26, 2025
Citation Information: J Clin Invest. 2025;135(21):e186652. https://doi.org/10.1172/JCI186652.×Abstract
The incretin receptor agonists semaglutide and tirzepatide have transformed the medical management of obesity. The neural mechanisms by which incretin analogs regulate appetite remain incompletely understood, and dissecting this process is critical for the development of next-generation antiobesity drugs that are more targeted and tolerable. Moreover, the physiologic functions of incretins in appetite regulation and gut-brain communication have remained elusive. Using in vivo fiber photometry, we discovered distinct pharmacologic and physiologic roles for the incretin hormones glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1). We showed that GIP, but not GLP-1, was required for normal nutrient-mediated inhibition of hunger-promoting AgRP neurons. By contrast, both GIP and GLP-1 analogs at pharmacologic doses were sufficient to inhibit AgRP neurons. The magnitude of neural inhibition was proportional to the effect of each incretin on food intake, and dual GIP and GLP-1 receptor agonism more potently inhibited AgRP neurons and suppressed food intake than either agonist alone. Our results have revealed a role for endogenous GIP in gut-brain appetite regulation and indicate that incretin analogs act in part via AgRP neurons to mediate their anorectic effects.
Authors
Hayley E. McMorrow, Andrew B. Cohen, Carolyn M. Lorch, Nikolas W. Hayes, Stefan W. Fleps, Joshua A. Frydman, Jessica L. Xia, Ricardo J. Samms, Lisa R. Beutler
Preeti Yadav, … , Kevin M. Tharp, Mallar BhattacharyaPreeti Yadav, … , Kevin M. Tharp, Mallar BhattacharyaPublished August 28, 2025
Citation Information: J Clin Invest. 2025;135(21):e188734. https://doi.org/10.1172/JCI188734.×Abstract
Idiopathic pulmonary fibrosis (IPF) is a disease of progressive lung remodeling and collagen deposition that leads to respiratory failure. Myeloid cells are abundant in IPF lung and in murine lung fibrosis, but their functional effects are incompletely understood. Using mouse and human lung models, we show that ornithine produced by myeloid cells expressing arginase 1 (ARG1) serves as a substrate for proline and collagen synthesis by lung fibroblasts. The predominant ARG1-expressing myeloid cells in mouse lung were macrophages, but in IPF lung, high-dimensional imaging revealed ARG1 was expressed mainly in neutrophils. Small-molecule ARG1 inhibition suppressed both ornithine levels and collagen expression in cultured, precision-cut IPF lung slices and in murine lung fibrosis. These results were confirmed in macrophage-specific Arg1-KO mice. Furthermore, we found that this pathway is regulated by cell-to-cell crosstalk, starting with purinergic signaling: extracellular ATP receptor P2RX4 was necessary for fibroblast IL-6 expression, which, in turn, was necessary for ARG1 expression by myeloid cells. Taken together, our findings define an immune-mesenchymal circuit that governs profibrotic metabolism in lung fibrosis.
Authors
Preeti Yadav, Javier Gómez Ortega, Prerna Dabral, Whitney Tamaki, Charles Chien, Kai-Chun Chang, Nivedita Biswas, Sixuan Pan, Julia Nilsson, Xiaoyang Yin, Aritra Bhattacharyya, Kaveh Boostanpour, Tanay Jujaray, Jasper T. Wang, Tatsuya Tsukui, Christopher J. Molina, Vincent C. Auyeung, Dean Sheppard, Baosheng Li, Mazharul Maishan, Hiroki Taenaka, Michael A. Matthay, Rieko Muramatsu, Lenka Maliskova, Arnab Ghosh, Walter L. Eckalbar, Ari B. Molofsky, Stanley J. Tamaki, Trever G. Bivona, Adam R. Abate, Allon Wagner, Satish K. Pillai, Paul J. Wolters, Kevin M. Tharp, Mallar Bhattacharya
Claire Leibler, … , Kevin M. Nickerson, Mark J. ShlomchikClaire Leibler, … , Kevin M. Nickerson, Mark J. ShlomchikPublished August 12, 2025
Citation Information: J Clin Invest. 2025;135(21):e189566. https://doi.org/10.1172/JCI189566.×Abstract
Toll like receptor (TLR) 7 and 9, endosomal sensors for RNA and DNA, are key mediators of autoreactivity. Although generally considered homologous, they paradoxically have opposing effects on lupus: TLR7 exacerbates the disease while TLR9 protects from it How they mediate opposing effects in autoimmunity remains undetermined. We hypothesized that differences in signaling qualities of the Toll-Interleukin 1 Receptor (TIR) domains of TLR7 and TLR9 could be responsible for their opposing effects. To test this, we introduced the TIR domain of TLR9 into the endogenous Tlr7 locus and the TLR7 TIR domain into the endogenous Tlr9 locus of mice, creating chimeric molecules termed TLR779 and TLR997. Lupus-prone MRL/lpr mice carrying Tlr779 had greatly ameliorated disease, while MRL/lpr mice carrying Tlr997 had markedly exacerbated disease compared with respective TlrWT mice. These experiments establish that TLR7 and TLR9 TIR domains have divergent properties and control disease quality, thus explaining the longstanding “TLR paradox”.
Authors
Claire Leibler, Kayla B. Thomas, Coralie Josensi, Russell C. Levack, Shuchi Smita, Shinu John, Daniel J. Wikenheiser, Sheldon Bastacky, Sebastien Gingras, Kevin M. Nickerson, Mark J. Shlomchik
Nina Martino, … , Pilar Alcaide, Alejandro P. AdamNina Martino, … , Pilar Alcaide, Alejandro P. AdamPublished September 16, 2025
Citation Information: J Clin Invest. 2025;135(21):e189570. https://doi.org/10.1172/JCI189570.×Abstract
Severe systemic inflammatory reactions, including sepsis, often lead to shock, organ failure, and death, in part through an acute release of cytokines that promote vascular dysfunction. However, little is known about the vascular endothelial signaling pathways regulating the transcriptional profile in failing organs. Our work focused on signaling downstream of IL-6, due to its clinical importance as a biomarker for disease severity and predictor of mortality. Here, we show that loss of endothelial expression of the IL-6 pathway inhibitor SOCS3 promoted a type I IFN–like (IFNI-like) gene signature in response to endotoxemia in mouse kidneys and brains. In cultured primary human endothelial cells, IL-6 induced transient IFNI-like gene expression in a noncanonical, IFN-independent fashion. We further show that STAT3, which we had previously demonstrated to control IL-6–driven endothelial barrier function, was dispensable for this activity. Instead, IL-6 promoted a transient increase in cytosolic mitochondrial DNA and required STAT1, cGAS, STING, and IRF1, -3, and -4. Inhibition of this pathway in endothelial cell–specific STING-KO mice or global STAT1-KO mice led to reduced the severity of the response to acute endotoxemic challenge and prevented expression of an endotoxin-induced IFNI-like gene signature. These results suggest that permeability and DNA-sensing responses are driven by parallel pathways downstream of this cytokine, provide potential insights into the complex response to acute inflammatory responses, and offer the possibility of novel therapeutic strategies for independently controlling the intracellular responses to IL-6 in order to tailor the inflammatory response.
Authors
Nina Martino, Erin K. Sanders, Ramon Bossardi Ramos, Iria Di John Portela, Fatma Awadalla, Shuhan Lu, Dareen Chuy, Neil Poddar, Mei Xing G. Zuo, Uma Balasubramanian, Peter A. Vincent, Pilar Alcaide, Alejandro P. Adam
Riikka Pietilä, … , Christer Betsholtz, Marie JeanssonRiikka Pietilä, … , Christer Betsholtz, Marie JeanssonPublished September 15, 2025
Citation Information: J Clin Invest. 2025;135(21):e190286. https://doi.org/10.1172/JCI190286.×Abstract
The role of endothelial dysfunction in tubulointerstitial fibrosis associated with chronic kidney disease (CKD) is not well understood. In this study, we demonstrate that the activation of the endothelial tyrosine kinase TIE2 alleviates renal pathology in experimental CKD in mice. TIE2 activation was achieved using a human angiopoietin-2–binding and TIE2-activating antibody (ABTAA) or through adult-induced endothelium-specific knockout of the vascular endothelial protein tyrosine phosphatase gene (Veptp). Both methods markedly protected CKD mice from endothelial dysfunction, peritubular capillary loss, tubular epithelial injury, and tubulointerstitial fibrosis. Conversely, silencing TIE2 through adult-induced endothelium-specific knockout of the Tie2 gene exacerbated CKD pathology. Additionally, we found that endothelial dysfunction promoted renal fibrosis not through endothelial-to-mesenchymal transition, as previously expected, but by inducing the expression of profibrotic PDGFB in tubular epithelial cells, a process that is inhibited by TIE2 activation. Our findings suggest that TIE2 activation via ABTAA warrants investigation as a therapy in human CKD, where there is a substantial unmet medical need.
Authors
Riikka Pietilä, Amanda Marks-Hultström, Liqun He, Sami Nanavazadeh, Susan E. Quaggin, Christer Betsholtz, Marie Jeansson
Yukun Guan, … , Seonghwan Hwang, Bin GaoYukun Guan, … , Seonghwan Hwang, Bin GaoPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e190635. https://doi.org/10.1172/JCI190635.×Abstract
Both adipocytes and hepatocytes have the capacity to store fat, but the factor(s) that determine fat distribution between these cell types remain unknown. In mice fed a high-fat diet, fat initially accumulates predominantly in adipocytes, while hepatic fat accumulation mainly emerges after the onset of epididymal adipocyte death that results in elevated free fatty acids to promote lipid accumulation in hepatocytes. However, it remains unclear whether other signals after adipocyte death are required to direct and/or promote hepatocytes to store fat and subsequently trigger metabolic dysfunction–associated steatotic liver disease (MASLD, formerly known as nonalcoholic fatty liver disease). Using genetically modified mouse models combined with bulk and single-cell RNA-Seq analysis, we demonstrated that visceral adipocyte death induced an accumulation of S100A8+ macrophages in the liver, which was partially induced by fatty acids and apoptotic adipocyte–derived extracellular vesicles. Macrophage-specific deletion of the S100a8 gene reduced hepatic fat accumulation and MASLD severity in mice. Mechanistically, S100A8+ macrophages suppressed cellular communication network factor 3 (CCN3), a negative regulator of CD36, thereby enhancing CD36 expression in hepatocytes. In conclusion, adipocyte death promotes hepatic infiltration of S100A8+ macrophages, which drive hepatocyte lipid storage and subsequently promote MASLD progression through CD36 upregulation, partially mediated by CCN3 suppression.
Authors
Yukun Guan, Yeonsoo Kim, Yang Wang, Ye Eun Cho, Xiaogang Xiang, Seung-Jin Kim, Tiantian Yao, Dechun Feng, Seonghwan Hwang, Bin Gao
Kelly L. Damm-Ganamet, … , Daniel B. Graham, Jacqueline PerrigoueKelly L. Damm-Ganamet, … , Daniel B. Graham, Jacqueline PerrigouePublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e191096. https://doi.org/10.1172/JCI191096.×Abstract
Solute carrier (SLC) transporters govern the selective transport of diverse molecules across cell membranes, controlling fundamental metabolic and cellular processes. Despite genetic evidence implicating SLC transporters in a variety of human diseases, this family of proteins represents an underexplored target class for therapeutic drug discovery. Here, we discovered a selective potentiator of SLC39A8, a metal transporter associated with inflammatory bowel disease, schizophrenia, and cardiovascular and metabolic disorders. We conducted a drug repurposing screen, identifying efavirenz as a potentiator of manganese and cadmium uptake by SLC39A8 and subsequently generated structure-activity relationships to guide design of analogs. Computational pocket identification methodology and molecular dynamic simulations revealed a ligandable, cryptic pocket that, together with functional mutagenesis, indicated direct target engagement and allosteric modulation. Our findings demonstrate how the combination of experimental data and computational tools represents a powerful synergy that can enhance scientific outcomes. This integrated approach allowed for iterative feedback where insights from experiments informed the model refinements and computational predictions guided future experimental designs. Furthermore, our data established that SLC39A8 transporter activity can be increased pharmacologically, potentially opening avenues for SLC transporter drug discovery.
Authors
Kelly L. Damm-Ganamet, Clara Moon, Alan D. Wickenden, Mark Tichenor, Yunhui Ge, Eduardo V. Mercado-Marin, Brian Chiou, Ayla Manughian-Peter, Taraneh Mirzadegan, Jennifer D. Venable, Ramnik J. Xavier, Jennifer E. Towne, Daniel B. Graham, Jacqueline Perrigoue
Shadisadat Esmaeili, … , Stephen J. Polyak, Joshua T. SchifferShadisadat Esmaeili, … , Stephen J. Polyak, Joshua T. SchifferPublished September 11, 2025
Citation Information: J Clin Invest. 2025;135(21):e192052. https://doi.org/10.1172/JCI192052.×Abstract
Molnupiravir is an antiviral medicine that induces lethal copying errors during SARS-CoV-2 RNA replication. Molnupiravir reduced hospitalization in one pivotal trial by 50% and had variable effects on reducing viral RNA levels in three separate trials. We used mathematical models to simulate these trials and closely recapitulated their virologic outcomes. Model simulations suggested lower antiviral potency against pre-Omicron SARS-CoV-2 variants than against Omicron. We estimated that in vitro assays underestimated in vivo potency by 6- to 7-fold against Omicron variants. Our model suggested that because polymerase chain reaction detects molnupiravir mutated variants, the true reduction in non-mutated viral RNA was underestimated by approximately 0.4 log10 in the two trials conducted while Omicron variants dominated. Viral area under the curve estimates differed significantly between non-mutated and mutated viral RNA. Our results reinforce past work suggesting that in vitro assays are unreliable for estimating in vivo antiviral drug potency and suggest that virologic endpoints for respiratory virus clinical trials should be catered to the drug mechanism of action.
Authors
Shadisadat Esmaeili, Katherine Owens, Ugo Avila-Ponce de Leon, Joseph F. Standing, David M. Lowe, Shengyuan Zhang, James A. Watson, William H.K. Schilling, Jessica Wagoner, Stephen J. Polyak, Joshua T. Schiffer
Gabriel Casella, … , Maryellen L. Giger, Marcus R. ClarkGabriel Casella, … , Maryellen L. Giger, Marcus R. ClarkPublished September 4, 2025
Citation Information: J Clin Invest. 2025;135(21):e192669. https://doi.org/10.1172/JCI192669.×Abstract
BACKGROUND In human lupus nephritis (LuN), tubulointerstitial inflammation (TII) is prognostically more important than glomerular inflammation. However, a comprehensive understanding of both TII complexity and heterogeneity is lacking.METHODS Herein, we used high-dimensional confocal microscopy, spatial transcriptomics, and specialized computer vision techniques to quantify immune cell populations and localize these within normal and diseased renal cortex structures. With these tools, we compared LuN to renal allograft rejection (RAR) and normal kidney tissues on 54 deidentified biopsies.RESULTS In both LuN and RAR, the 33 characterized immune cell populations formed discrete subgroups whose constituents covaried in prevalence across biopsies. In both diseases, these covariant immune cell subgroups organized into the same unique niches. Therefore, inflammation could be resolved into trajectories representing the relative prevalence and density of cardinal immune cell members of each covariant subgroup. Indeed, in any one biopsy, the inflammatory state could be characterized by quantifying constituent immune cell trajectories. Remarkably, LuN heterogeneity could be captured by quantifying a few myeloid immune cell trajectories, while RAR was more complex with additional T cell trajectories.CONCLUSIONS Our studies identify rules governing renal inflammation and thus provide an approach for resolving LuN into discrete mechanistic categories.FUNDING NIH (U19 AI 082724 [MRC], R01 AI148705 [MRC and ASC]), Chan Zuckerberg Biohub (MRC), and Lupus Research Alliance (MRC).
Authors
Gabriel Casella, Madeleine S. Torcasso, Junting Ai, Thao P. Cao, Satoshi Hara, Michael S. Andrade, Deepjyoti Ghosh, Daming Shao, Anthony Chang, Kichul Ko, Anita S. Chong, Maryellen L. Giger, Marcus R. Clark
Ge Dong, … , Ying Fu, Zhigang CaiGe Dong, … , Ying Fu, Zhigang CaiPublished September 4, 2025
Citation Information: J Clin Invest. 2025;135(21):e193011. https://doi.org/10.1172/JCI193011.×Abstract
Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome is a hemato-rheumatoid disease caused by somatic UBA1 mutations in hematopoietic stem cells (HSCs). The pathogenic cell type(s) responsible for the syndrome are unknown, and murine models recapitulating the disease are lacking. We report that loss of Uba1 in various mouse hematopoietic cell types resulted in pleiotropic consequences and demonstrate that an approximate 70% loss of Uba1 in neutrophils (NEs) of murine mutants induced nonlethal VEXAS-like symptoms. Depletion of Uba1 in HSCs induced extensive hematopoietic cell loss, whereas depletion of Uba1 in B cells, T cells, or megakaryocytes induced corresponding cell death, but these mutant mice appeared normal. Depletion of Uba1 in monocytes and NEs failed to induce cell death, and the mutant mice were viable. Among the tested models, only depletion of Uba1 in NEs induced autoinflammatory symptoms including increased counts and percentages of NEs, increased proinflammatory cytokines, presence of vacuoles in myeloid cells, splenomegaly, and dermatitis. Residual Uba1 was approximately 30% in the mutant NEs, which disrupted cellular hemostasis. Finally, genetic loss of the myeloid prosurvival regulator Morrbid partially mitigated the VEXAS-like symptoms. The established VEXAS-like murine model will further our understanding and treatment of the newly identified autoinflammatory syndrome prevalent among aged men.
Authors
Ge Dong, Jingjing Liu, Wenyan Jin, Hongxi Zhou, Yuchen Wen, Zhiqin Wang, Keyao Xia, Jianlin Zhang, Linxiang Ma, Yunxi Ma, Lorie Chen Cai, Qiufan Zhou, Huaquan Wang, Wei Wei, Ying Fu, Zhigang Cai
Gisela Gabernet, … , Leying Guan, Lauren I.R. EhrlichGisela Gabernet, … , Leying Guan, Lauren I.R. EhrlichPublished September 9, 2025
Citation Information: J Clin Invest. 2025;135(21):e193698. https://doi.org/10.1172/JCI193698.×Abstract
BACKGROUND Following SARS-CoV-2 infection, approximately 10%–35% of patients with COVID-19 experience long COVID (LC), in which debilitating symptoms persist for at least 3 months. Elucidating the biologic underpinnings of LC could identify therapeutic opportunities.METHODS We utilized machine learning methods on biologic analytes provided over 12 months after hospital discharge from more than 500 patients with COVID-19 in the IMPACC cohort to identify a multiomics “recovery factor,” trained on patient-reported physical function survey scores. Immune profiling data included PBMC transcriptomics, serum O-link and plasma proteomics, plasma metabolomics, and blood mass cytometry by time of flight (CyTOF) protein levels. Recovery factor scores were tested for association with LC, disease severity, clinical parameters, and immune subset frequencies. Enrichment analyses identified biologic pathways associated with recovery factor scores.RESULTS Participants with LC had lower recovery factor scores compared with recovered participants. Recovery factor scores predicted LC as early as hospital admission, irrespective of acute COVID-19 severity. Biologic characterization revealed increased inflammatory mediators, elevated signatures of heme metabolism, and decreased androgenic steroids as predictive and ongoing biomarkers of LC. Lower recovery factor scores were associated with reduced lymphocyte and increased myeloid cell frequencies. The observed signatures are consistent with persistent inflammation driving anemia and stress erythropoiesis as major biologic underpinnings of LC.CONCLUSION The multiomics recovery factor identifies patients at risk of LC early after SARS-CoV-2 infection and reveals LC biomarkers and potential treatment targets.TRIAL REGISTRATION ClinicalTrials.gov NCT04378777.FUNDING National Institute of Allergy and Infectious Diseases (NIAID), NIH (3U01AI167892-03S2, 3U01AI167892-01S2, 5R01AI135803-03, 5U19AI118608-04, 5U19AI128910-04, 4U19AI090023-11, 4U19AI118610-06, R01AI145835-01A1S1, 5U19AI062629-17, 5U19AI057229-17, 5U19AI057229-18, 5U19AI125357-05, 5U19AI128913-03, 3U19AI077439-13, 5U54AI142766-03, 5R01AI104870-07S1, 3U19AI089992-09, 3U19AI128913-03, and 5T32DA018926-1, 3U19AI1289130, U19AI128913-04S1, R01AI122220); NIH (UM1TR004528); and National Science Foundation (NSF) (DMS2310836).
Authors
Gisela Gabernet, Jessica Maciuch, Jeremy P. Gygi, John F. Moore, Annmarie Hoch, Caitlin Syphurs, Tianyi Chu, Naresh Doni Jayavelu, David B. Corry, Farrah Kheradmand, Lindsey R. Baden, Rafick-Pierre Sekaly, Grace A. McComsey, Elias K. Haddad, Charles B. Cairns, Nadine Rouphael, Ana Fernandez-Sesma, Viviana Simon, Jordan P. Metcalf, Nelson I. Agudelo Higuita, Catherine L. Hough, William B. Messer, Mark M. Davis, Kari C. Nadeau, Bali Pulendran, Monica Kraft, Chris Bime, Elaine F. Reed, Joanna Schaenman, David J. Erle, Carolyn S. Calfee, Mark A. Atkinson, Scott C. Brakenridge, Esther Melamed, Albert C. Shaw, David A. Hafler, Alison D. Augustine, Patrice M. Becker, Al Ozonoff, Steven E. Bosinger, Walter Eckalbar, Holden T. Maecker, Seunghee Kim-Schulze, Hanno Steen, Florian Krammer, Kerstin Westendorf, IMPACC Network, Bjoern Peters, Slim Fourati, Matthew C. Altman, Ofer Levy, Kinga K. Smolen, Ruth R. Montgomery, Joann Diray-Arce, Steven H. Kleinstein, Leying Guan, Lauren I.R. Ehrlich
Yrina Rochman, … , Drew Weissman, Marc E. RothenbergYrina Rochman, … , Drew Weissman, Marc E. RothenbergPublished September 23, 2025
Citation Information: J Clin Invest. 2025;135(21):e194080. https://doi.org/10.1172/JCI194080.×Abstract
Allergic diseases have reached epidemic proportions globally, calling attention to the need for better treatment and preventive approaches. Herein, we developed allergen-encoding messenger RNA (mRNA)–lipid nanoparticle (LNP) strategies for both therapy and prevention of allergic responses. Immunization with allergen-encoded mRNA-LNPs modulated T cell differentiation, inhibiting the generation of T helper type 2 and type 17 cells upon allergen exposure in experimental asthma models induced by ovalbumin, and naturally occurring house dust mite (HDM) and the major HDM allergen Der p1. Allergen-specific mRNA-LNP treatment attenuated clinicopathology in both preventive and established allergy models, including reduction in eosinophilia, mucus production, and airway hypersensitivity, while enhancing production of allergen-specific IgG antibodies and maintaining low IgE levels. Additionally, allergen-specific mRNA-LNP vaccines in mice elicited a CD8+CD38+KLRG– T cell response as seen following SARS-CoV-2 mRNA vaccination in humans, underscoring a conserved immune mechanism across species, regardless of the mRNA-encoded protein. Notably, mRNA-LNP vaccination in combination with an mTOR inhibitor reduced the CD8+ T cell response without affecting the vaccine-induced anti-allergic effect in the preventive model of asthma. This technology renders allergen-specific mRNA-LNP therapy a promising approach for prevention and treatment of allergic diseases.
Authors
Yrina Rochman, Michael Kotliar, Andrea M. Klingler, Mark Rochman, Mohamad-Gabriel Alameh, Jilian R. Melamed, Garrett A. Osswald, Julie M. Caldwell, Jennifer M. Felton, Lydia E. Mack, Julie Hargis, Ian P. Lewkowich, Artem Barski, Drew Weissman, Marc E. Rothenberg
Beibei Liu, … , Da Li, Yingqun HuangBeibei Liu, … , Da Li, Yingqun HuangPublished August 12, 2025
Citation Information: J Clin Invest. 2025;135(21):e194879. https://doi.org/10.1172/JCI194879.×Abstract
Through a combination of single-cell/single-nucleus RNA-Seq (sc/snRNA-Seq) data analysis, immunohistochemistry, and primary macrophage studies, we have identified pathogenic macrophages characterized by Tet methylcytosine dioxygenase 3 (TET3) overexpression (Toe-Macs) in 3 major human diseases associated with chronic inflammation: metabolic dysfunction–associated steatohepatitis (MASH), non–small cell lung cancer (NSCLC), and endometriosis. These macrophages are induced by common factors present in the disease microenvironment (DME). Crucially, the universal reliance on TET3 overexpression among these macrophages enabled their selective elimination as a single population, irrespective of heterogeneity in other molecular markers. In mice, depleting these macrophages via myeloid-specific Tet3 KO markedly mitigated disease progression, and the therapeutic effects were recapitulated pharmacologically using a TET3-specific small-molecule degrader. Through an unexpected mode of action, TET3 epigenetically regulated the expression of multiple genes key to the generation and maintenance of an inflammatory/immunosuppressive DME. We propose that Toe-Macs are a unifying feature of pathogenic macrophages that could be therapeutically targeted to treat MASH, NSCLC, endometriosis, and potentially other chronic inflammatory diseases.
Authors
Beibei Liu, Yangyang Dai, Zixin Wang, Jiahui Song, Yushu Du, Haining Lv, Stefania Bellone, Yang-Hartwich Yang, Andrew Kennedy, Songying Zhang, Muthukumaran Venkatachalapathy, Yulia Surovtseva, Penghua Wang, Gordon G. Carmichael, Hugh S. Taylor, Xuchen Zhang, Da Li, Yingqun Huang
Michael H. Kramer, … , Christopher A. Miller, Timothy J. LeyMichael H. Kramer, … , Christopher A. Miller, Timothy J. LeyPublished August 7, 2025
Citation Information: J Clin Invest. 2025;135(21):e195929. https://doi.org/10.1172/JCI195929.×Abstract
Mutations that initiate acute myeloid leukemia (AML) can cause clonal expansion without transformation (clonal hematopoiesis). Cooperating mutations, usually in signaling genes, are needed to cause overt disease, but these may require a specific fitness state to be tolerated. Here, we show that nearly all AMLs arising in a mouse model expressing 2 common AML-initiating mutations (Dnmt3aR878H and Npm1cA) acquired a single copy amplification of chromosome 7 (chr7), followed by activating mutations in signaling genes. We show that overexpression of a single gene on chr7 (Gab2, which coordinates signaling pathways) was tolerated in the presence of the Npm1cA mutation, could accelerate the development of AML, and was important for the survival of fully transformed AML cells. GAB2 is likewise overexpressed in many human AMLs with mutations in NPM1 and/or signaling genes, and also in acute promyelocytic leukemia initiated by PML::RARA; the PML::RARA fusion protein may activate GAB2 by directly binding to its 5′ flanking region. A similar pattern of GAB2 overexpression preceding mutations in signaling genes has been described in other human malignancies. GAB2 overexpression may represent an oncogene-driven adaptation that facilitates the action of signaling mutations, suggesting an important (and potentially targetable) missing link between the initiating and progression mutations associated with AML.
Authors
Michael H. Kramer, Stephanie N. Richardson, Yang Li, Tiankai Yin, Nichole M. Helton, Daniel R. George, Michelle Cai, Sai Mukund Ramakrishnan, Casey D.S. Katerndahl, Christopher A. Miller, Timothy J. Ley
-
Corrigenda
Charlotte Gehin, … , Giovanni D’Angelo, Vincenzo A. GennarinoCharlotte Gehin, … , Giovanni D’Angelo, Vincenzo A. GennarinoPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e200195. https://doi.org/10.1172/JCI200195.×Abstract
Authors
Charlotte Gehin, Museer A. Lone, Winston Lee, Laura Capolupo, Sylvia Ho, Adekemi M. Adeyemi, Erica H. Gerkes, Alexander P.A. Stegmann, Estrella López-Martín, Eva Bermejo-Sánchez, Beatriz Martínez-Delgado, Christiane Zweier, Cornelia Kraus, Bernt Popp, Vincent Strehlow, Daniel Gräfe, Ina Knerr, Eppie R. Jones, Stefano Zamuner, Luciano A. Abriata, Vidya Kunnathully, Brandon E. Moeller, Anthony Vocat, Samuel Rommelaere, Jean-Philippe Bocquete, Evelyne Ruchti, Greta Limoni, Marine Van Campenhoudt, Samuel Bourgeat, Petra Henklein, Christian Gilissen, Bregje W. van Bon, Rolph Pfundt, Marjolein H. Willemsen, Jolanda H. Schieving, Emanuela Leonardi, Fiorenza Soli, Alessandra Murgia, Hui Guo, Qiumeng Zhang, Kun Xia, Christina R. Fagerberg, Christoph P. Beier, Martin J. Larsen, Irene Valenzuela, Paula Fernández-Álvarez, Shiyi Xiong, Robert Śmigiel, Vanesa López-González, Lluís Armengol, Manuela Morleo, Angelo Selicorni, Annalaura Torella, Moira Blyth, Nicola S. Cooper, Valerie Wilson, Renske Oegema, Yvan Herenger, Aurore Garde, Ange-Line Bruel, Frederic Tran Mau-Them, Alexis B.R. Maddocks, Jennifer M. Bain, Musadiq A. Bhat, Gregory Costain, Peter Kannu, Ashish Marwaha, Neena L. Champaigne, Michael J. Friez, Ellen B. Richardson, Vykuntaraju K. Gowda, Varunvenkat M. Srinivasan, Yask Gupta, Tze Y. Lim, Simone Sanna-Cherchi, Bruno Lemaitre, Toshiyuki Yamaji, Kentaro Hanada, John E. Burke, Ana Marija Jakšić, Brian D. McCabe, Paolo De Los Rios, Thorsten Hornemann, Giovanni D’Angelo, Vincenzo A. Gennarino
Li-Bing Song, … , Yi-Xin Zeng, Mu-Sheng ZengLi-Bing Song, … , Yi-Xin Zeng, Mu-Sheng ZengPublished November 3, 2025
Citation Information: J Clin Invest. 2025;135(21):e200436. https://doi.org/10.1172/JCI200436.×Abstract
Authors
Li-Bing Song, Jun Li, Wen-Ting Liao, Yan Feng, Chun-Ping Yu, Li-Juan Hu, Qing-Li Kong, Li-Hua Xu, Xing Zhang, Wan-Li Liu, Man-Zhi Li, Ling Zhang, Tie-Bang Kang, Li-Wu Fu, Wen-Lin Huang, Yun-Fei Xia, Sai Wah Tsao, Mengfeng Li, Vimla Band, Hamid Band, Qing-Hua Shi, Yi-Xin Zeng, Mu-Sheng Zeng



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)