Advertisement
ResearchIn-Press PreviewNephrologyVascular biology
Open Access |  10.1172/JCI190286
10.1172/JCI190286
TIE2 activation by antibody-clustered endogenous angiopoietin-2 prevents capillary loss and fibrosis in experimental kidney disease
Riikka Pietilä,1 Amanda M. Marks-Hultström,1 Liqun He,1 Sami Nanavazadeh,1 Susan E. Quaggin,2 Christer Betsholtz,1 and Marie Jeansson3
1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by Pietilä, R. in: PubMed | Google Scholar
1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by Marks-Hultström, A. in: PubMed | Google Scholar
1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by
He, L.
in:
PubMed
|
Google Scholar
|

1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by Nanavazadeh, S. in: PubMed | Google Scholar
1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by
Quaggin, S.
in:
PubMed
|
Google Scholar
|

1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by
Betsholtz, C.
in:
PubMed
|
Google Scholar
|

1Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
2Feinberg Cardiovascular Research Institute and Division of Nephrology and H, Northwestern University, Chicago, United States of America
3Department of Medicine Huddinge, Karolinska Institutet, Huddinge, Sweden
Find articles by
Jeansson, M.
in:
PubMed
|
Google Scholar
|

Published September 15, 2025 - More info
Copyright © 2025, Pietilä et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
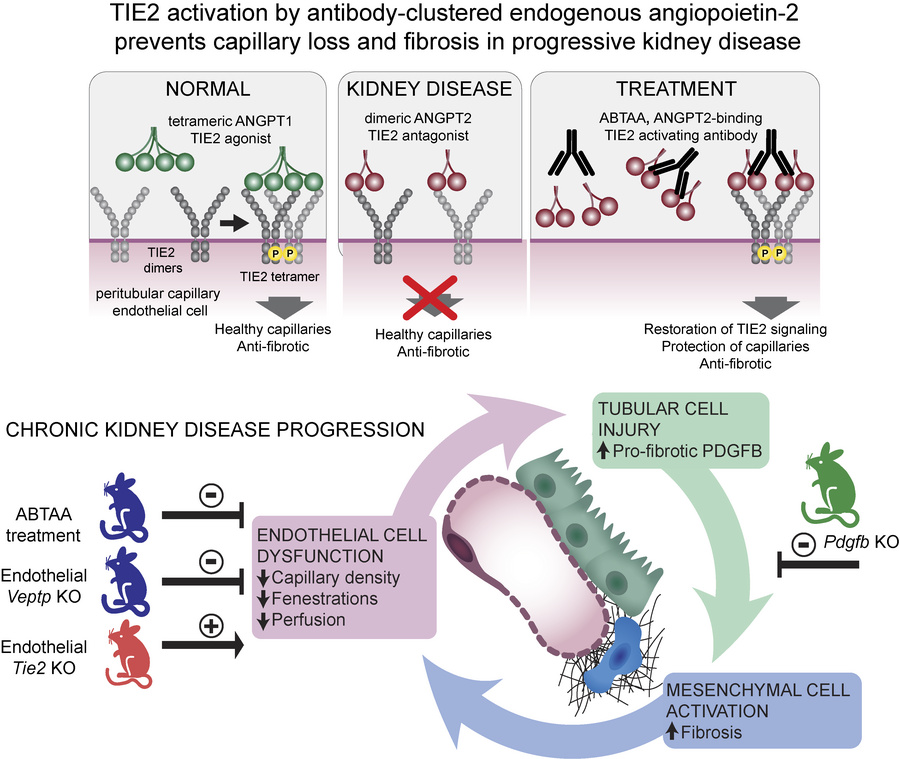
The role of endothelial dysfunction in tubulointerstitial fibrosis associated with chronic kidney disease (CKD) is not well understood. In this study, we demonstrate that the activation of the endothelial tyrosine kinase TIE2 alleviates renal pathology in experimental CKD in mice. TIE2 activation was achieved using a human angiopoietin-2 (ANGPT2)-binding and TIE2-activating antibody (ABTAA), or through adult-induced endothelial-specific knockout of the vascular endothelial protein tyrosine phosphatase gene (Veptp). Both methods significantly protected CKD mice from endothelial dysfunction, peritubular capillary loss, tubular epithelial injury, and tubulointerstitial fibrosis. Conversely, silencing TIE2 through adult-induced endothelial-specific knockout of the Tie2 gene exacerbated CKD pathology. Additionally, we found that endothelial dysfunction promotes renal fibrosis not through endothelial-to-mesenchymal transition as previously expected, but by inducing the expression of pro-fibrotic PDGFB in tubular epithelial cells, a process that is inhibited by TIE2 activation. Our findings suggest that TIE2 activation via ABTAA warrants investigation as a therapy in human CKD, where there is a substantial unmet medical need.
-
Version history
- Version 1 (September 15, 2025): In-Press Preview



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)