Abstract
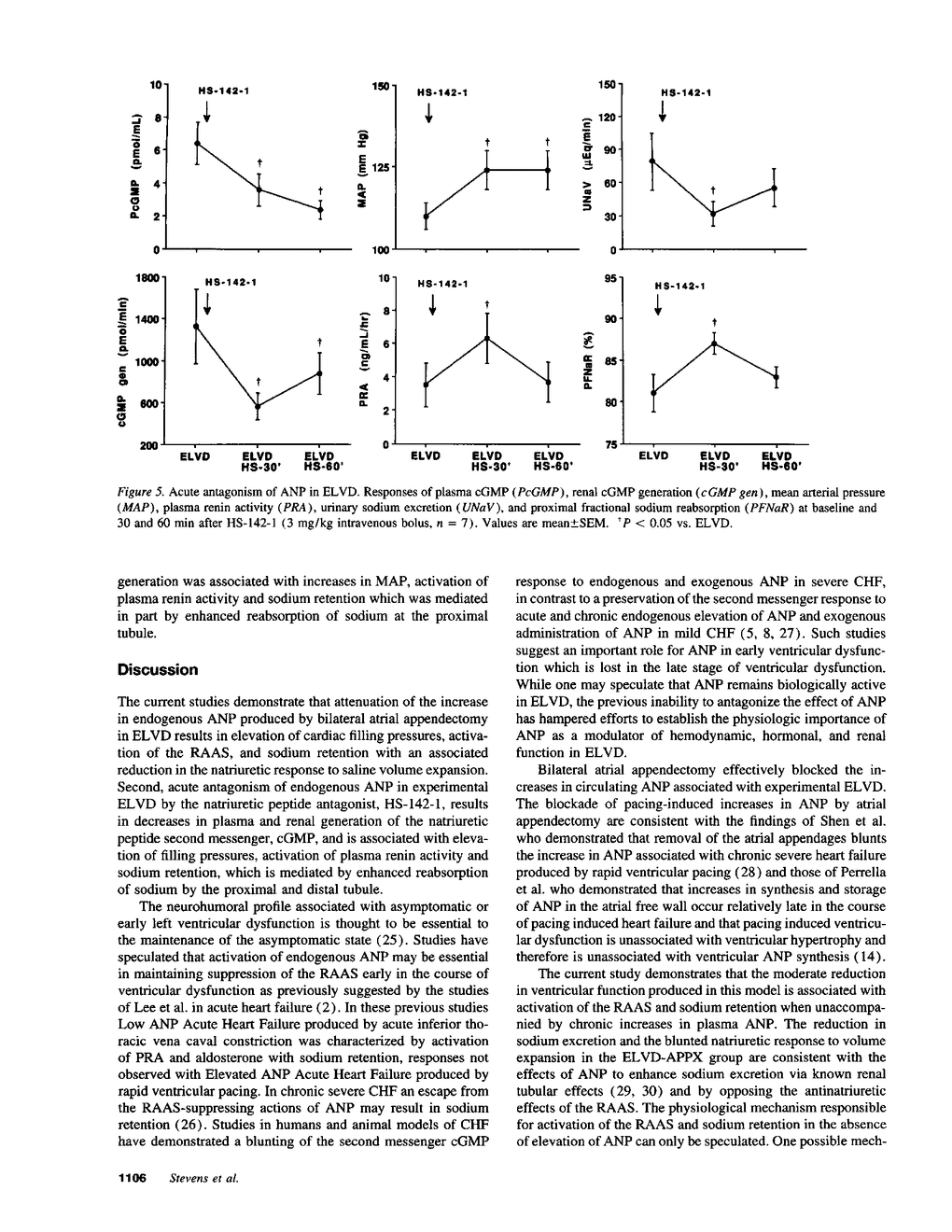
Asymptomatic or early left ventricular dysfunction in humans is characterized by increases in circulating atrial natriuretic peptide (ANP) without activation of the renin-angiotensin-aldosterone system (RAAS). We previously reported a canine model of early left ventricular dysfunction (ELVD) produced by rapid ventricular pacing and characterized by an identical neurohumoral profile and maintenance of the natriuretic response to volume expansion (VE). To test the hypothesis that elevated endogenous ANP suppresses the RAAS and maintains sodium excretion in ELVD, we assessed the effects of antagonism of ANP on cardiorenal and neurohumoral function in ELVD. Chronic ANP suppression was produced by bilateral atrial appendectomies before the production of ELVD by rapid ventricular pacing (ELVD-APPX, n = 5). This group was compared with a separate group with ELVD and intact atrial appendages (ELVD-INTACT, n = 8). ELVD-APPX was characterized by lower circulating ANP (50 +/- 11 vs. 158 +/- 37 pg/ml, P < 0.05), activation of plasma renin activity (PRA) (9.4 +/- 2.4 vs. 0.6 +/- 0.4 ng/ml per h, P < 0.05) and aldosterone (36.4 +/- 12.5 vs. 2.5 +/- 0.0 ng/dl, P < 0.05) when compared to ELVD-INTACT. In comparison to the ELVD-INTACT group, sodium excretion was decreased before and during VE in the ELVD-APPX group. Acute ANP antagonism was produced by administration of the particulate guanylate cyclase coupled natriuretic peptide receptor antagonist, HS-142-1, to seven conscious dogs with ELVD and intact atrial appendages (ELVD-INTACT). HS-142-1 decreased plasma concentrations and renal generation of the ANP second messenger, cGMP, and was associated with activation of PRA and sodium retention with enhanced tubular sodium reabsorption. These data support a significant role for elevated endogenous ANP in the maintenance of sodium excretion and regulation of the RAAS in experimental ELVD.
Authors
T L Stevens, J C Burnett Jr, M Kinoshita, Y Matsuda, M M Redfield
Other pages:
| 1101 | 1102 | 1103 | 1104 | 1105 | 1106 | 1107 | 1108 |