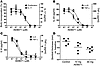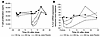Advertisement
Research ArticleDermatology Free access | 10.1172/JCI35636
The PKC inhibitor AEB071 may be a therapeutic option for psoriasis
Hans Skvara,1 Markus Dawid,1 Elise Kleyn,2 Barbara Wolff,3 Josef G. Meingassner,3 Hilary Knight,4 Thomas Dumortier,4 Tamara Kopp,1 Nasanin Fallahi,1 Georg Stary,1 Christoph Burkhart,4 Olivier Grenet,4 Juergen Wagner,4 Youssef Hijazi,4 Randall E. Morris,4 Claire McGeown,4 Christiane Rordorf,4 Christopher E.M. Griffiths,2 Georg Stingl,1 and Thomas Jung4
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Skvara, H. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Dawid, M. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Kleyn, E. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Wolff, B. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Meingassner, J. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Knight, H. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Dumortier, T. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Kopp, T. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Fallahi, N. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Stary, G. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Burkhart, C. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Grenet, O. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Wagner, J. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Hijazi, Y. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Morris, R. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by McGeown, C. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Rordorf, C. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Griffiths, C. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Stingl, G. in: PubMed | Google Scholar
1Department of Dermatology, Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, Vienna, Austria. 2Dermatological Sciences, Salford Royal NHS Foundation Trust, University of Manchester, Manchester, United Kingdom. 3Institute for Biomedical Research, Novartis, Vienna, Austria. 4Institute for Biomedical Research and Institute for Exploratory Development, Novartis, Basel, Switzerland.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
Find articles by Jung, T. in: PubMed | Google Scholar
Published August 7, 2008 - More info
J Clin Invest. 2008;118(9):3151–3159. https://doi.org/10.1172/JCI35636.
© 2008 The American Society for Clinical Investigation
Received: March 14, 2008; Accepted: June 25, 2008
-
Abstract
PKC isoforms t, α, and β play fundamental roles in the activation of T cells and other immune cell functions. Here we show that the PKC inhibitor AEB071 both abolishes the production of several cytokines by activated human T cells, keratinocytes, and macrophages in vitro and inhibits an acute allergic contact dermatitis response in rats. To translate these findings into humans, single and multiple ascending oral doses of AEB071 were administered to healthy volunteers and patients with psoriasis, respectively. AEB071 was well tolerated with no clinically relevant laboratory abnormalities. Ex vivo stimulation of lymphocytes from subjects exposed to single doses of AEB071 resulted in a dose-dependent inhibition of both lymphocyte proliferation and IL2 mRNA expression. Clinical severity of psoriasis was reduced up to 69% compared with baseline after 2 weeks of treatment, as measured by the Psoriasis Area Severity Index (PASI) score. The improvement in psoriasis patients was accompanied by histological improvement of skin lesions and may be partially explained by a substantial reduction of p40+ dermal cells, which are known to mediate psoriasis. These data suggest that AEB071 could be an effective novel treatment regimen for psoriasis and other autoimmune diseases, and that AEB071 warrants long-term studies to establish safety and efficacy.
-
Introduction
PKC isoforms have been shown to play key roles in cellular signaling, proliferation, differentiation, migration, survival, and death. In resting cells, PKCs are predominantly localized in the cytosol and are catalytically inactive due to autoinhibition by their pseudosubstrate domain. Upon cell activation, PKC isotype–specific signals trigger translocation from the cytosol to the membrane and induce conformational changes, which displace the pseudosubstrate moiety from the catalytic domain and enable PKC isotypes to phosphorylate specific protein substrates (1). Most isoforms are ubiquitously expressed, except PKCγ and PKCθ. While PKCγ is exclusively found in the brain, high protein levels of PKCθ are seen predominantly in hematopoietic cells and skeletal muscle. PKCα and PKCθ as well as PKCβ and PKCδ are functionally important for T and B cell signaling, respectively (2–4). PKCθ plays an essential role in T cell activation because it is the only isoform that is selectively translocated to the T cell/antigen-presenting cell contact site immediately after cell-cell interaction (5). Furthermore, PKCθ is crucial for IL-2 production, a prerequisite for the proliferation of T cells (6). PKCθ-deficient mice are defective in NF-κB activation (7) and are resistant to experimental autoimmune encephalomyelitis, probably due to impaired production of IFN-γ and IL-17 (8). PKCα in T cells is required for proliferation and IFN-γ production (9). B cells require PKCβ for proper antigen receptor function and PKCδ for the induction of tolerance (4). Thus, PKC isoforms in T and B cells are considered attractive therapeutic targets for autoimmune diseases and transplantation (10).
AEB071 is to our knowledge a novel PKC inhibitor that has strong and specific activity on PKCθ, PKCα, and PKCβ and lesser activity on PKCδ, PKCε, and PKCη, suggesting that AEB071 would inhibit not only T cells, but also a variety of other cells. It is selective for more than 200 other kinases, including those important for early T cell activation, such as lck and ZAP-70. A clinical proof of concept strategy addressing this complex inhibitory profile was needed to demonstrate safety and efficacy in humans. In particular, patients with a disease driven mostly by T cells and in part by resident cells were thought to benefit most from such an approach. This is the case in psoriasis, a chronic, currently incurable autoimmune skin disease defined by clinical presentation of red, heavily scaled skin plaques containing dense infiltrates of T cells, macrophages, and dendritic cells as well as hyperproliferation and incomplete differentiation of epidermal keratinocytes (11). While there is strong evidence that skin-infiltrating T cells play a crucial role in driving the psoriatic process (11–13), more recent data generated in preclinical models reemphasize that skin-resident cells, such as keratinocytes, expressing the PKC isoforms α, δ, ε, η, and ζ (14) may contribute to the pathogenesis (15, 16). Here we report results of a translational medicine effort to demonstrate clinical proof of concept in humans. We show that orally administered AEB071 inhibited activation of peripheral blood T cells from AEB071-exposed human volunteers in a dose-dependent manner and that clinical signs and symptoms of psoriasis significantly improved during the course of a 2-week clinical study.
-
Results
AEB071 is a potent inhibitor of classical and novel PKC isotypes. It does not inhibit other kinases involved in cell signaling, including fyn, lck, ZAP-70, and JAK3 (J. Wagner, unpublished observations). KI values are in the picomolar range for the PKC isotypes α (0.95 nM), β (0.64 nM), and θ (0.22 nM). AEB071 also inhibits the PKC isoforms δ, ε, and η, with KI values between 1.8 and 3.2 nM. In vitro, AEB071 showed a strong effect on T cell proliferation (Figure 1A) and cytokine production by anti-CD3/anti-CD28-activated T cells. IL-17 (Figure 1A), IFN-γ, IL-2, and TNF-α were inhibited with IC50 values below 100 nM (data not shown). Human blood monocyte-derived macrophages were responsive to AEB071 and were inhibited to produce IL-23 (IC50, 16 nM) and TNF-α (IC50, 74 nM) (Figure 1B). Very similar results were observed with monocyte-derived dendritic cells (data not shown). Also, IL-8 and TNF-α production by activated human keratinocytes was inhibited with IC50 values of about 1 nM (Figure 1C). In addition, orally administered AEB071 dose dependently and significantly inhibited an acute allergic contact dermatitis reaction in rats (Figure 1D). Thus, AEB071 shows a pharmacological profile in vitro and in vivo consistent with its ability to inhibit various PKC isoforms in cells potentially involved in human skin inflammatory disorders.
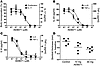 Figure 1
Figure 1AEB071 inhibits T cell proliferation, cytokine production in vitro, and acute contact dermatitis in vivo. (A) Proliferation of T cells induced by stimulation of PBMCs with soluble anti-CD3 and anti-CD28 mAbs. Results from 1 of 4 experiments are shown. IC50, 108 nM. IL-17 production under the same conditions, IC50 84 nM (1 of 2 experiments). (B) Inhibition of IL-23 (IC50, 16 nM) and TNF-α (IC50, 74 nM) production by zymosan-activated human macrophages. Data are from 1 of 2 experiments. (C) Inhibition of IL-8 (IC50, 2.8 nM) and TNF-α (IC50, 1.4 nM) production in PMA-activated normal human keratinocytes. (D) Inhibition of rat allergic contact dermatitis by oral dosing with 10 mg (P = 0.055 versus vehicle control) and 30 mg (P = 0.002 versus vehicle control) of AEB071 as assessed by flank skin thickening in groups of 5 animals each. Data are mean ± SD.
AEB071 administered to human volunteers as a single dose up to 500 mg is well tolerated and inhibits ex vivo PKC-dependent proliferation and IL-2 production by activated T cells
To investigate general safety and tolerability, a total of 48 healthy volunteers were recruited. Six subjects per cohort were treated with ascending single oral doses of AEB071 (10, 25, 50, 100, 200, and 500 mg), and 12 subjects were administered placebo. Overall, AEB071 was well tolerated with a total of 12 dose-independent adverse events (AEs) reported, none of which was serious. These included headache, night sweats, dyspepsia, nausea, and dizziness in the active dose groups. One adverse event (diarrhea) was reported for a placebo-treated subject. A reversible increase in the mean ventricular heart rate was observed at a dose level of 500 mg AEB071 compared with placebo. The increase in heart rate (7% to 23%, ECG data) was transient and lasted from 3 to 12 hours after dose administration, peaking at 6 hours after administration. However, mean heart rates were still within the upper limit of the normal range and therefore were not considered as abnormal tachycardia. Peripheral blood was sampled at intervals in order to investigate PKC-dependent T cell functions ex vivo. As shown in Figure 2A, there was a dose-dependent inhibition of T cell proliferation induced by the mitogen PHA peaking between 1 and 3 hours after oral dosing and returning to baseline levels 12 hours after dose. Similarly, levels of IL2 mRNA induced by the PKC activator PMA and costimulated by anti-CD28 were reduced maximally at 3 hours after dose (Figure 2B). These pharmacodynamic activities correlated with AEB071 drug levels in peripheral blood.
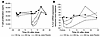 Figure 2
Figure 2Ex vivo analysis of peripheral blood from healthy volunteers exposed to AEB071. (A) Peripheral blood was taken from subjects exposed to single doses of 100, 200, or 500 mg AEB071 or placebo and stimulated with phytohemagglutinin. Proliferation was measured by [3H]-thymidine incorporation. Data show the geometric mean of 6 subjects per cohort in cpm. Time point 0 reflects the blood sample taken before dosing. To establish the variation at baseline, blood samples were taken at time points –24, –23, –20, and –18 hours on the day before dosing. (B) Peripheral blood was taken similarly as described in A and stimulated with PMA and anti-CD28 antibodies. IL2 mRNA levels were measured by quantitative RT-PCR. Data show the geometric mean of 6 subjects per cohort as the percent IL2 mRNA reduction compared with baseline.
As shown in Table 1, the maximum blood levels of AEB071 were on average 1.1, 2, and 4 μM at oral doses of 100, 200, and 500 mg, respectively, and occurred between 1 and 5 hours after dose. The elimination half-life was about 6 hours. The exposure, AUC, and maximum plasma concentration (Cmax) increased in an approximate dose-related manner over the dose ranges tested. The IC50 levels were calculated to be 0.87 μM for T cell proliferation and 0.97 μM for IL2 mRNA inhibition. These concentrations were achieved at Cmax following a single dose of 100 mg AEB071 (Table 1). It is of note that at the same exposure (~1 μM), proliferation of T cells in vitro was completely inhibited (Figure 1A), most probably due to the high serum protein binding property (>95%) of AEB071, which resulted in lower bioactivity in vivo as compared with in vitro conditions. Together, these data demonstrate that oral administration of AEB071 was well tolerated and resulted in blood concentrations high enough to produce a dose-dependent pharmacodynamic effect on PKC-dependent T cell function. Therefore, it was hypothesized that AEB071 might be effective in a disease associated with T cell activation such as psoriasis.
AEB071 improves psoriasis at well-tolerated doses within a 2-week treatment period
A total of 32 patients with moderate to severe plaque psoriasis were enrolled in 4 cohorts of 8 patients each. Median (range) age of all patients was 41 yr (20–65 yr), weight was 82.75 kg (60–103 kg), and BMI was 27.15 kg/m2 (19.5–34.7 kg/m2). Six patients were dosed with 25, 100, 200, or 300 mg AEB071 twice daily (bid), resulting in a total dose of 50, 200, 400, and 600 mg AEB071 per day for 2 weeks in a dose-escalating fashion. Median (range) Psoriasis Area Severity Index (PASI; ref. 17) scores at baseline were 11.3 (4.5–19.6), 13.05 (5.8–22.8), 10.55 (4.2–16.5), and 14.9 (9.3–19.5), respectively. In each cohort, 2 patients received placebo in a double-blind, randomized manner, and median PASI score for the placebo cohort at baseline was 11.65 (range, 3.6–19). After completion of day 8 dosing of each cohort, a review of the safety and tolerability findings was performed prior to the decision to allow the next higher dose cohort to commence. Patients were followed up for 2 consecutive weeks following cessation of treatment with either AEB071 or placebo.
Safety and tolerability. Similar to the single-dose healthy volunteer study, multiple-dose treatment for 2 weeks with AEB071 was well tolerated with no serious adverse events. The incidence of AEs in the active treatment groups was 45.8%, compared with 37.5% in the placebo group. The observed AEs were mild in intensity, and none resulted in any patient discontinuing from the study. Nausea (in 300 mg bid cohort) was the only AE in the active groups that affected 2 of the 6 subjects. There was no AE that showed a dose response. Clinical laboratory values were unaltered in the majority of patients, with no evidence of clinically significant changes compared with baseline in any of the treatment groups. A nonsignificant increase of alanine aminotransferase (ALT; twice the normal upper limit) occurred in 1 patient in the 200 mg bid group at days 10 and 12. The ALT levels started to decline before the end of the treatment phase (day 14) and returned to baseline levels by the first follow-up visit (day 21). A second patient experienced a similar elevation of ALT on day 6 in the 300 mg bid cohort, which returned to baseline despite further treatment. Renal function as assessed by serum creatinine values and creatinine clearance was found to be normal during the entire study for all patients. Pulse rate, blood pressure, and QT interval data remained stable and within the normal range throughout the trial period.
Clinical and histological improvement in psoriasis. Clinical severity of psoriasis was assessed by PASI scoring (17) on a weekly basis for 4 weeks. A dose-dependent improvement of psoriasis was observed during the 2-week treatment period (Figure 3). The mean reduction of PASI scores over baseline was 69% for the 300 mg bid cohort (placebo, 5.3%) with 4 of 6 patients having achieved a PASI75 (equivalent to a 75% or more improvement in PASI over baseline). This mean reduction was significantly different from the reduction in placebo controls (95% CI for the mean difference versus placebo, [42%, 85%]; Table 2). The mean reduction in the 300 mg bid group had already achieved significance versus placebo after 1 week (95% CI for the mean difference versus placebo, [5%, 48%]). Figure 4 shows a psoriatic plaque at baseline and after 2 weeks of therapy with AEB071 300 mg bid. During the 2-week follow-up period in which no active psoriasis treatment was allowed, most patients demonstrated a relapse of their disease and almost reached baseline PASI levels. Only patients in the 300 mg bid cohort still showed improvement over baseline. There were no rebound events, i.e., rapid worsening of the disease as compared with baseline.
 Figure 3
Figure 3Clinical improvement of psoriasis as assessed by PASI scoring. Six subjects per cohort of 25, 100, 200, and 300 mg bid AEB071 and 8 subjects in the placebo group were treated for 2 weeks and followed up for another 2 weeks in the absence of any relevant psoriatic treatment. Data show mean ± SEM as percent PASI reduction compared with baseline. A detailed statistical analysis is given in Table 2.
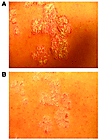 Figure 4
Figure 4Improvement of psoriasis during treatment with AEB071 in a representative patient. (A) Typical plaque with scaling, erythema, and induration before treatment (baseline) and (B) after administering AEB071 300 mg bid for 2 weeks.
A cutaneous infiltrate of T cells accompanied by proliferation and incomplete differentiation of keratinocytes are hallmarks of psoriasis histopathology. Immunohistological evidence of improvement was observed with 100 and 200 mg bid doses (data not shown), but significant changes were seen only in the 300 mg bid cohort (Figure 5). Figure 5A summarizes the findings for dermal CD3+ T cells in a 2-week biopsy indicating a marked reduction of T cells following treatment. The number of proliferating keratinocytes (Ki-67+) was significantly reduced after 2 weeks of treatment (Figure 5B). Consequently, epidermal thickness (Figure 5C) and expression of the differentiation antigen K16 (data not shown) returned to normal after treatment. Although statistically not significant, CD207+ epidermal Langerhans cells repopulated the epidermis as a sign of disease improvement (18) (Figure 5C). A nonsignificant reduction of dermal CD14+ monocytes and CD15+ neutrophils was observed (data not shown). IL-12/IL-23p40+ but not IL-1β+ cells were found to be significantly reduced after 1 week of treatment as compared with baseline (Figure 5D). These results demonstrate that 2 weeks of treatment with AEB071 at the highest administered dose of 300 mg bid was highly effective in normalizing cutaneous histology in psoriasis patients.
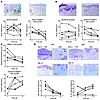 Figure 5
Figure 5Immunohistological analyses show improvement of psoriasis following AEB071 treatment. (A) Representative slides (original magnification, ×100) stained for T cells (CD3+) are shown at baseline (left) and after treatment with 300 mg bid (right). The corresponding plots show the number of dermal T cells at baseline and days 7 and 14 for the placebo (n = 8, left) and the AEB071 300 mg bid cohort (n = 6, right) (paired t test, P = 0.001, day 14 compared with baseline). (B) Representative slides (original magnification, ×100) stained for proliferating cells (Ki-67+) is shown at baseline (left) and after 300 mg bid treatment (right). The corresponding plots show the number of Ki-67+ epidermal cells at baseline and days 7 and 14 for the placebo (n = 8, left) and the 300 mg bid cohort (n = 6, right) (paired t test, P = 0.002, day 14 compared with baseline). (C) Epidermal thickening (left) in μm (paired t test, P = 0.011, day 14 compared with baseline) and number of CD207+ Langerhans cells in the epidermis (right) are shown for the 300 mg bid cohort. (D) Immunohistological staining of p40+ and IgG1+ cells at baseline (day 0) and day 14 of treatment with 300 mg bid AEB071 in a single subject. Plots for dermal p40+ and IL-1β+ cells show individual data for patients treated with 300 mg bid AEB071 (n = 5). P = 0.0015, day 7 compared with baseline, and P = 0.0002, day 14 compared with baseline, for p40+ cells (paired t test).
Pharmacokinetics. AEB071 was rapidly absorbed, with time to Cmax (Tmax) of about 2 hours, which was in line with expectations from the first study in healthy volunteers (Table 3). Exposure with AEB071 was almost dose proportional. The Cmax reached a mean of 4.5 μM at steady state after 300 mg bid dosing, which was clinically highly effective and consistent with the pharmacodynamic data from the single-dose study that showed marked inhibition of T cells at this dose. The minimum plasma concentration (Cmin) was about 1.5 μM at 300 mg bid, higher than the IC50 values for inhibition of proliferation and IL2 mRNA expression. Clinical improvement was already evident at 200 mg bid, corresponding to a Cmin of about 0.7 μM and a Cmax of about 2.6 μM. Skin penetration of AEB071 was investigated at steady state. As shown in Table 4, the mean skin concentration of AEB071 ranged between 0.28 and 3.3 μM, dependent upon the dose given. A similar exposure in skin as compared with blood was observed in the 300 mg bid group. Steady state was achieved within 1 day, with no accumulation after multiple dosing.
-
Discussion
In this study the results of introducing a PKC inhibitor (AEB071) in humans are presented. The pharmacological profile of AEB071 indicates its potential to inhibit a variety of cells involved in the pathogenesis of autoimmune diseases. Preclinical in vitro profiling suggested that AEB071 was active on cells mediating psoriasis, a hypothesis further supported by the demonstration of its activity in a skin inflammatory animal model. A surrogate biomarker for PKC activity was chosen to show the inhibitory activity of AEB071 on T cells in a first in man safety and tolerability study which encouraged us to conduct a short-term trial in patients with psoriasis as a model for autoimmune diseases. This strict scientific approach resulted in a preliminary risk/benefit assessment along with a first understanding of the activity of AEB071 at a cellular level.
To the best of our knowledge, AEB071 is currently the only PKC inhibitor in the exploratory phase of drug development for autoimmune diseases. There are other PKC inhibitors in early clinical development for non-autoimmune indications. Based on the observation that PKC is activated by hyperglycemia and the preferential activation of PKCβ in the retina, the PKCβ-specific inhibitor LY333531 (ruboxistaurin) is in development for diabetic retinopathy (19). Another PKC inhibitor is enzastaurin, which targets not only PKCβ but also other kinases (20). This multi-kinase inhibitor and other less selective PKC inhibitors are currently in early phase I trials in oncology (21).
In vitro, AEB071 had a strong effect on T cells, which confirms the hypothesis that a PKC inhibitor predominantly blocking PKCα, PKCθ, and PKCβ isoforms expressed in T cells would also inhibit T cell functions. In particular, IL-17, highly upregulated in psoriasis skin (22, 23) and functionally a proinflammatory cytokine for keratinocytes in vitro (24) and in vivo (25), was potently inhibited by AEB071. The fact that orally administered AEB071 to rats inhibited an acute T cell–driven contact dermatitis model further confirmed that AEB071 had a strong effect on T cells. A dose-dependent inhibition of T cell proliferation and IL2 mRNA production by AEB071 following a PKC-dependent activation pathway was demonstrated during the first phase I trial in human healthy volunteers. Although cellular PKC activity could not be measured in these studies, as specific targets of PKC isoforms have not yet been identified, IL-2 production and proliferation of T cells serve as surrogate biomarkers for PKC activity. Since T cells play a critical role in psoriasis (12, 26–31), the effect of AEB071 in a multiple-dose regimen in psoriasis patients was studied. Clinically, a dose-dependent improvement of psoriasis was observed reaching a mean 69% reduction in PASI score after 2 weeks of treatment with 300 mg bid AEB071. In this cohort, 4 of 6 patients achieved a PASI75. In similarly small proof of concept studies, the anti–TNF-α antibody infliximab induced an approximate mean 40% reduction in PASI after 2 weeks (as assessed in 11 patients treated with the highest tested dose of 10 mg/kg) over baseline (32). Further, an anti–IL-12/IL-23p40 antibody produced a mean 35%–40% improvement after 2 weeks in a study with 5 psoriasis patients treated with the highest tested dose of 5 mg/kg (33). Both these new biologic therapies target cytokines fundamentally important for the pathogenesis of psoriasis (12, 34–36). In contrast, biologic therapies targeting only T cells have a slower and less robust onset of action as demonstrated by the less than 20% improvement in psoriasis after 2 weeks when targeting CD2 (29) or CD80/86 (28). Similar to TNF-α or IL-12/IL-23p40 inhibitors, AEB071 showed a rapid and robust onset of action, indicating that it may have additional effects on skin-resident cells. This is supported by immunohistological analyses. After 1 week of treatment, there was a trend for improvement, and after 2 weeks the skin was histologically almost normal in those subjects treated with the highest dose. AEB071 was very potent in reducing the number of p40+ dermal cells. Interestingly, the reduction of p40+-expressing cells was evident already after 1 week, thus preceding other histological as well as clinical criteria of treatment responses. Although this leaves open whether IL-12, IL-23, or both of these p40-sharing cytokines are inhibited, it suggests that AEB071 may inhibit both the Th1 as well as the Th17 pathway, both of which are activated in psoriasis (22, 37). Thus, p40 reduction together with inhibition of TNF-α production may sufficiently explain the mechanism by which AEB071 induces a strong clinical response. The fact that IL-1β production by cells in the dermis and by activated macrophages in vitro was not inhibited (IC50 of 10 μM in 3 experiments) suggests that IL-1β does not play a major role in psoriasis. However, a formal proof has not been provided yet.
Besides T cells, dendritic cells, and macrophages, keratinocytes may play a major role in psoriasis pathogenesis. Ligands of the vitamin D or retinoid receptor induce differentiation of epidermal cells and are effective in the treatment of psoriasis (38, 39). Transgenic mice overexpressing epidermal VEGF (15) or STAT3 (16) develop clinical and histological lesions typical of psoriasis. In humans, overexpression of VEGF and phosphorylated STAT3 are features of psoriasis (16, 40). PKC isoforms expressed in keratinocytes may contribute to these changes. STAT3 has been shown to be phosphorylated at Ser727 residues by PKCε, and PKCε transgenic mice demonstrate phosphorylation of both Ser727 and Tyr705 residues and therefore display fully activated STAT3 (41). Keratinocyte-specific overexpression of PKCα in mice results in expression of TNF-α and VEGF and epidermal neutrophilic inflammation (42, 43), i.e., characteristic features of psoriasis. This K5-PKCα transgenic mouse requires phorbol-ester activation to induce a strong cutaneous inflammation in which IL-8 is overexpressed and which can be specifically blocked by a PKCα inhibitor (44). Consistent with this, we found that phorbol-ester–induced IL-8 and TNF-α production by keratinocytes were completely blocked by AEB071, indicating that AEB071 indeed inhibits PKC activity in activated human keratinocytes.
T cells may be either activated in lymph nodes and subsequently infiltrate into the skin or be activated by dendritic cells and/or keratinocytes (45) locally in the skin. It is not known how sensitive skin-infiltrating T cells are to inhibition by AEB071, compared with peripheral resting T cells. Data presented here suggest that skin exposure of about 1 μM achieved with 200 mg bid was associated with clinical efficacy. In contrast, a peak blood exposure of about 1 μM AEB071, which resulted in a 50% inhibition of T cell proliferation and IL2 mRNA expression, was achieved with a single dose of 100 mg AEB071, a dose that was clinically only minimally effective. This suggests that preactivated skin-infiltrating T cells are less sensitive to AEB071 compared with resting T cells.
In conclusion, this report describes what is to our knowledge the first study of a PKC inhibitor in an autoimmune disease. AEB071’s pharmacological ability to inhibit various PKC isoforms with different potency and to be selective for PKC over other kinases makes it a potential candidate to treat effectively a number of autoimmune diseases. Given the ubiquitous expression of PKC and the associated potential safety concerns, the tolerability and absence of toxic events in the presented trials is encouraging for further studies to establish long-term safety and clinical efficacy in psoriasis and other autoimmune diseases.
-
Methods
In vitro experiments
Human PBMCs isolated from buffy coats were incubated with graded concentrations of AEB071 and stimulated for 72 hours with anti-CD3 (clone SPV-T3/1, isotype IgG2a, prepared at Novartis Institute for Biomedical Research) and anti-CD28 (BD Biosciences) at a concentration of 1 μg/ml each. To determine proliferation of cells, a BrdU incorporation assay (Roche) was used. IL-17 production was analyzed after 24 hours of culture by ELISA (R&D Systems). Primary human keratinocytes isolated from breast tissue after reductive surgery were cultured in Keratinocyte Growth Medium (Clonetics) supplemented with 10% heat-inactivated FCS (HyClone), 1 mM glutamine, 100 U/ml penicillin/100 μg/ml streptomycin (both from Gibco), and 0.06 mM calcium. Cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich) for 24 hours in the presence of AEB071, and supernatants were analyzed for IL-8 and TNF-α by ELISA. Human macrophages were prepared from monocytes that had been isolated from buffy coats by MACS. Monocytes were incubated with GM-CSF for a total of 7 days, with a medium change after 3 days. Cells were stimulated for 24 hours with 1 mg/ml zymosan suspension (Sigma-Aldrich) in the presence of AEB071. Supernatants were analyzed for TNF-α and IL-23 levels by ELISA. All in vitro experiments were carried out with triplicate samples.
Allergic contact dermatitis in rats
This study was performed according to study protocol MA 58-3260/03, approved by the Landesregierung Wien, Magistratsabteilung 58, Vienna, Austria. Sensitization of Crl:CD(SD) rats was initiated with 80 μl 2% 2,4-dinitrofluorobenzene (DNFB; Merck; dissolved in 50% acetone, 10% DMSO, and 38% olive oil, vol/vol), which was applied in volumes of 20 μl to the inner surface of both ear lobes and to both shaved inguinal regions on day 1. On day 12, allergic contact dermatitis was elicited on test sites (15 mm in diameter) on both shaved flanks with 30 μl DNFB (0.5% in 80% acetone, 19.5% olive oil, vol/vol). Changes in skin thickness as a measure of skin inflammation before and 24 hours after the challenge were recorded. The animals were treated with an oral gavage 1 hour before and 4 hours after the challenge. Changes in skinfold thickness in compound- and vehicle-treated animals were compared and the data analyzed by t test. For practical reasons, the animals were sedated for handling with isofluorane.
First in human single and multiple-dose studies
Study CAEW334A2101 was a single-center randomized, double-blind, parallel-group, time-lagged, single-oral-dose study in healthy subjects, using a placebo control and 6 ascending dose levels of AEB071 (10, 25, 50, 100, 200, and 500 mg). The study was conducted in Allschwil, Switzerland, through Swiss Pharma Contract Ltd. after approval of the ethics committees of the Medical University of Basel (Basel, Switzerland) and the Swiss health authority (Swissmedic, Bern, Switzerland). A total of 48 subjects entered and completed the study; 36 received the drug and 12 received the placebo. Adverse events were recorded throughout the study. Holter-ECGs were performed at baseline and study day 1. Blood pressure and ECG were recorded at predose, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, and 72 hours after dose. Laboratory parameters were obtained 3, 24, 48, and 72 hours after dose.
Pharmacodynamic assay for lymphocyte proliferation in whole blood. Proliferation of T cells in whole, anticoagulated blood after phytohemagglutinin ex vivo stimulation was determined by [3H]-thymidine incorporation. Human whole blood (20 μl) was supplemented with 180 μl X-Vivo10 medium (BioWhittaker Europe) containing 3.3 mg/ml phytohemagglutinin. In general, 6 replicate wells were prepared. After 48 hours, wells were pulsed with [3H]-thymidine (1 μCi/well) overnight and radioactivity was measured in a BETAPLATE Liquid Scintillation Counter (Wallac).
Stimulation of whole blood and detection of IL2 mRNA. Blood (270 μl) was activated in duplicates with a 30-μl PMA/anti-CD28 cocktail for 6 hours at 37°C/5% CO2. The final concentration of PMA and anti-CD28 antibody was 14 ng/ml and 1 μg/ml, respectively. Stimulated blood (200 μl) was incubated with 700 μl of lysis buffer and subsequently kept at –80°C. Total RNA was extracted and reverse transcribed to cDNA according to standard procedures. Real-time PCR was performed using ABI Prism 7900HT (Applied Biosystems) in combination with TaqMan PCR Master Mix (Applied Biosystems; catalog no. 4304437). TaqMan assays were available in Applied Biosystems database and were purchased: GAPDH (catalog Hs99999905_m1) and IL-2 (catalog Hs00174114_m1). The PCR conditions consisted of one cycle at 50°C for 2 min followed by 1 cycle of denaturation at 95°C for 10 min and 40 cycles of amplification, a denaturation step at 95°C for 15 s, and an annealing/elongation step at 60°C for 1 min. A relative quantification of mRNA was performed using a standard curve for the housekeeping gene (Ambion; catalog no. 7976). The efficiency of the reaction was calculated from the slope of the standard curve: efficiency = 10 – 1 / slope – 1. Because each dilution of standard cDNA had a known concentration, the quantity (copy number) of cDNA in each sample could be determined, according to the CT values. The normalization of the samples was performed by calculating the ratio of expression levels of the gene of interest to those of the housekeeping gene.
Study CAEB071A2101 was a 2-week multiple ascending dose, double-blind placebo-controlled study to evaluate the safety, tolerability, and pharmacokinetics of twice daily administration of oral AEB071 and to explore the pharmacodynamics of oral AEB071 in moderate to severe psoriasis patients. The study was conducted at 2 sites, in Vienna, Austria, and Manchester, United Kingdom, and was approved by the local ethics committees (of the Medical University of Vienna and the Medical University of Manchester) and health authorities (Bundesministerium für Gesundheit, Vienna, Austria, and Medicines and Healthcare Products Regulatory Agency, London, United Kingdom). Patients aged between 18 and 65 years with stable plaque psoriasis and no other clinically significant abnormalities were enrolled. Patients were hospitalized for study weeks 1 and 2 and were further closely monitored as outpatients on a weekly basis during study weeks 3 and 4. Four consecutive cohorts of 8 patients each were treated with oral AEB071 for 2 weeks with rising doses (6 patients in each cohort) or with placebo (2 patients in each cohort). Escalating doses of AEB071 (25, 100, 200, and 300 mg bid) were administered. The start of the next higher dose level was permitted only after demonstration of tolerability and safety at the preceding lower dose. Blood pressure, pulse rate, ECG evaluations, and hematology/blood chemistry laboratory parameters were closely monitored from day –1 and throughout the study period, including the follow-up period at days 21 and 28. Creatinine clearance was determined at days –1 and 14. Disease severity was assessed on a weekly basis using PASI (17), a validated score widely used in clinical research in psoriasis. Skin biopsies (5 mm) were taken from typical psoriatic plaques from all patients at baseline (day 0), day 7, and day 14 (end of treatment period). Epidermal thickening and the enumeration of CD3+, CD14+, CD15+, CD207+, and Ki-67+ cells together with a K16 evaluation were analyzed as described previously (46).
Immunohistochemical cytokine staining. Frozen tissue was cut into 5-μm sections and mounted on capillary gap microscope slides (Dako). To determine the distribution and the number of IL-1β+ or p40+ cells, single immunostainings were performed (anti-p40: clone 31052, mIgG1; R&D; anti–IL-1β: clone 2D8, mIgG1; Abcam). The cryostat sections were air-dried for 20 min, fixed in ice-cold acetone for 10 min, and either stained immediately or stored at –20°C. An additional fixation step was performed with 2% paraformaldehyde (Sigma-Aldrich) in PBS for 5 min. After washing with 0.05% Tween (Bio-Rad Laboratories) in PBS (Gibco), the slides were blocked with 1% BSA and 0.05% Tween in PBS and, after washing with 0.05% Tween in PBS, with 5% horse serum and 1% saponine (Sigma-Aldrich) in PBS to prevent nonspecific protein binding. An IgG1 isotype monoclonal antibody was used as negative control. To detect the primary antibody, a biotinylated horse anti-mouse IgG (Vector Laboratories) followed by incubation with avidin-peroxidase was used. For visualization of the positively stained cells, 0.01% 3-amino-9-ethyl-carbazol in 50 mM acetate buffer (pH 5, 0.015% H2O 2.5% dimethylformamide) was used as chromogen. Sections were counterstained with hematoxylin.
Pharmacokinetic assessments
For the single-dose study in healthy volunteers, blood samples were obtained prior to dose and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48, 72, and 96 hours after dose. For the psoriasis study, blood samples were obtained before dose and 1, 2, 4, 6, 8, and 12 hours after the morning dose on day 1 and day 14. Cmin levels were also determined before the first morning dose on days 2, 4, 6, 8, 10, and 12. A skin biopsy was taken from a typical psoriatic lesion from all patients on day 14 approximately 4 hours after the morning dose. AEB071 concentrations were analyzed by LC/MS with a limit of quantitation of 3 ng/ml (blood) and 12 ng/g (skin). Pharmacokinetics data analysis was conducted using a standard non-compartmental approach, and parameters were derived using WinNonlin 5.0 software (Pharsight).
Statistics
In the single-dose study, the pharmacodynamic evaluations were summarized by means of descriptive statistics. The percent inhibition of lymphocyte proliferation and IL2 mRNA expression compared with baseline was similarly summarized. In the multiple-dose study, the percent inhibition compared with baseline in the PASI score was analyzed by means of a linear mixed-effect model adjusted for the baseline level, the treatment group, the study day, and the study day–by–treatment group interaction. For each AEB071 dose, the estimate for the mean difference versus placebo (and associated 95% CI) was obtained from the model at each week. The change from baseline in skin histology endpoints was analyzed similarly. Baseline levels were compared with post-baseline levels using a 2-tailed paired t test at a 5% significance level. The incidence of AEs was also summarized.
-
Acknowledgments
We thank Gerda Herzig and Nicole Schöfmann for providing excellent technical help for the in vitro studies and Josiane Bringel and Magali Marcellin for RNA extraction and IL2 mRNA measurements.
Address correspondence to: Thomas Jung, Translational Medicine Dermatology, Novartis Exploratory Development, Forum 1, WSJ-27.4.071, CH-4002 Basel, Switzerland. Phone: 41-61-3247340; Fax: 41-61-3240810; E-mail: thomas.jung@novartis.com.
-
Footnotes
Nonstandard abbreviations used: AE, adverse event; Cmax, maximum plasma concentration; PASI, Psoriasis Area Severity Index.
Conflict of interest: This study was sponsored by Novartis Exploratory Development and Novartis Institutes for Biomedical Research. B. Wolff, J.G. Meingassner, H. Knight, T. Dumortier, C. Burkhart, O. Grenet, J. Wagner, Y. Hijazi, R.E. Morris, C. McGeown, C. Rordorf, and T. Jung are Novartis employees. C.E.M. Griffiths and G. Stingl have previously received consultancy fees from Novartis.
Reference information: J. Clin. Invest.118:3151–3159 (2008). doi:10.1172/JCI35636
-
References
- Newton, A.C. 2003. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 370:361-371.
- Spitaler, M., Cantrell, D.A. 2004. Protein kinase C and beyond. Nat. Immunol. 5:785-790.
- Guo, B., Su, T.T., Rawlings, D.J. 2004. Protein kinase C family functions in B-cell activation. Curr. Opin. Immunol. 16:367-373.
- Mecklenbrauker, I., Saijo, K., Zheng, N.Y., Leitges, M., Tarakhovsky, A. 2002. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 416:860-865.
- Monks, C.R., Kupfer, H., Tamir, I., Barlow, A., Kupfer, A. 1997. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 385:83-86.
- Bauer, B., et al. 2000. T cell expressed PKCtheta demonstrates cell-type selective function. Eur. J. Immunol. 30:3645-3654.
- Manicassamy, S., Gupta, S., Sun, Z. 2006. Selective function of PKC-theta in T cells. Cell Mol. Immunol. 3:263-270. View this article via: PubMed Google Scholar
- Tan, S.L., et al. 2006. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C theta-deficient mice. J. Immunol. 176:2872-2879. View this article via: PubMed Google Scholar
- Pfeifhofer, C., et al. 2006. Defective IgG2a/2b class switching in PKC alpha–/– mice.
J. Immunol. 176:6004-6011. View this article via: PubMed Google Scholar
- Chaudhary, D., Kasaian, M. 2006. PKCtheta: A potential therapeutic target for T-cell-mediated diseases. Curr. Opin. Investig. Drugs. 7:432-437. View this article via: PubMed Google Scholar
- Griffiths, C.E., Barker, J.N. 2007. Pathogenesis and clinical features of psoriasis. Lancet. 370:263-271.
- Lowes, M.A., Bowcock, A.M., Krueger, J.G. 2007. Pathogenesis and therapy of psoriasis. Nature. 445:866-873.
- Nickoloff, B.J., Nestle, F.O. 2004. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113:1664-1675.
- Breitkreutz, D., et al. 2007. Protein kinase C family: On the crossroads of cell signaling in skin and tumor epithelium. J. Cancer Res. Clin. Oncol. 133:793-808.
- Xia, Y.P., et al. 2003. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 102:161-168.
- Sano, S., et al. 2005. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 11:43-49.
- Fredriksson, T., Pettersson, U. 1978. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 157:238-244. View this article via: PubMed Google Scholar
- Gordon, K.B., Bonish, B.K., Patel, T., Leonardi, C.L., Nickoloff, B.J. 2005. The tumour necrosis factor-alpha inhibitor adalimumab rapidly reverses the decrease in epidermal Langerhans cell density in psoriatic plaques. Br. J. Dermatol. 153:945-953.
- Clarke, M., Dodson, P.M. 2007. PKC inhibition and diabetic microvascular complications. Best Pract. Res. Clin. Endocrinol. Metab. 21:573-586.
- Herbst, R.S., Oh, Y., Wagle, A., Lahn, M. 2007. Enzastaurin, a protein kinase Cbeta- selective inhibitor, and its potential application as an anticancer agent in lung cancer. Clin. Cancer Res. 13:s4641-s4646.
- Serova, M., et al. 2006. Preclinical and clinical development of novel agents that target the protein kinase C family. Semin. Oncol. 33:466-478.
- Haider, A.S., et al. 2008. Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J. Immunol. 180:1913-1920. View this article via: PubMed Google Scholar
- Lowes, M.A., et al. 2008. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest Dermatol. 128:1207-1211.
- Teunissen, M.B., Koomen, C.W., de Waal, M.R., Wierenga, E.A., Bos, J.D. 1998. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111:645-649.
- Zheng, Y., et al. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 445:648-651.
- Gottlieb, S.L., et al. 1995. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat. Med. 1:442-447.
- Krueger, J.G., et al. 2000. Successful in vivo blockade of CD25 (high-affinity interleukin 2 receptor) on T cells by administration of humanized anti-Tac antibody to patients with psoriasis. J. Am. Acad. Dermatol. 43:448-458.
- Abrams, J.R., et al. 1999. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J. Clin. Invest. 103:1243-1252.
- Ellis, C.N., Krueger, G.G. 2001. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N. Engl. J. Med. 345:248-255.
- Weinshenker, B.G., Bass, B.H., Ebers, G.C., Rice, G.P. 1989. Remission of psoriatic lesions with muromonab-CD3 (orthoclone OKT3) treatment. J. Am. Acad. Dermatol. 20:1132-1133. View this article via: PubMed Google Scholar
- Utset, T.O., et al. 2002. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J. Rheumatol. 29:1907-1913. View this article via: PubMed Google Scholar
- Chaudhari, U., et al. 2001. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 357:1842-1847.
- Kauffman, C.L., et al. 2004. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J. Invest. Dermatol. 123:1037-1044.
- Kleyn, C.E., Griffiths, C.E. 2006. Infliximab for the treatment of psoriasis. Expert. Opin. Biol. Ther. 6:797-805.
- Philipp, S., et al. 2006. The evaluation of psoriasis therapy with biologics leads to a revision of the current view of the pathogenesis of this disorder. Expert. Opin. Ther. Targets. 10:817-831.
- Nestle, F.O., Conrad, C. 2004. The IL-12 family member p40 chain as a master switch and novel therapeutic target in psoriasis. J. Invest. Dermatol. 123:xiv-xxv. View this article via: PubMed Google Scholar
- Lee, E., et al. 2004. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 199:125-130.
- Nagpal, S., Lu, J., Boehm, M.F. 2001. Vitamin D analogs: mechanism of action and therapeutic applications. Curr. Med. Chem. 8:1661-1679. View this article via: PubMed Google Scholar
- Johnson, A., Chandraratna, R.A. 1999. Novel retinoids with receptor selectivity and functional selectivity. Br. J. Dermatol. 140(Suppl. 54):12-17. View this article via: PubMed Google Scholar
- Detmar, M., et al. 1994. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J. Exp. Med. 180:1141-1146.
- Aziz, M.H., Manoharan, H.T., Verma, A.K. 2007. Protein kinase C epsilon, which sensitizes skin to sun’s UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3. Cancer Res. 67:1385-1394.
- Cataisson, C., Pearson, A.J., Torgerson, S., Nedospasov, S.A., Yuspa, S.H. 2005. Protein kinase C alpha-mediated chemotaxis of neutrophils requires NF-kappa B activity but is independent of TNF alpha signaling in mouse skin in vivo. J. Immunol. 174:1686-1692. View this article via: PubMed Google Scholar
- Cataisson, C., et al. 2003. Activation of cutaneous protein kinase C alpha induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J. Immunol. 171:2703-2713. View this article via: PubMed Google Scholar
- Cataisson, C., et al. 2006. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J. Clin. Invest. 116:2757-2766.
- Boyman, O., et al. 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 199:731-736.
- Bangert, C., Friedl, J., Stary, G., Stingl, G., Kopp, T. 2003. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Invest. Dermatol. 121:1409-1418.
-
Version history
- Version 1 (August 7, 2008): No description
- Version 2 (September 2, 2008): No description



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)